1. Introduction
2. Experimental Procedure
2.1. Chemicals
2.2. Anchoring monoatomic Cu to BaTiO3
2.3. Characterization and PE activity test
3. Results and Discussion
3.1. XRD analysis
3.2. Observations of TEM and HAADF-STEM
3.3. XPS analysis
3.4. Piezoelectric characteristics of the sample
3.5. Tetracycline degradation
3.6. Mechanism discussion
4. Conclusion
1. Introduction
Environmental water pollution and the discharge of toxic and hazardous organic pollutants have become more severe in recent years as a result of the rapid growth of industrialization. The accumulation of toxic organic and inorganic compounds has led to a degradation of the quality of water, soil, and air, resulting in environmental contamination in the modern world. This is a consequence of the development of global industrialization.1,2) Chemotherapeutic agents and antibiotics are used for the prevention and treatment of bacterial infections. Humans rely on them extensively because of their exceptional antimicrobial and anti-inflammatory properties. At extremely high concentrations, tetracycline, a pure antibiotic, rapidly restricts bacterial growth and disrupts the synthesis of bacterial proteins, resulting in the extinction of bacteria. Antibiotics have accumulated significantly in the environment due to their frequent and widespread abuse and use.3,4)
Hence, further studies into environmentally sustainable methods of protection are necessary to address the issues arising from dye wastewater. Energy conversion employing nanostructured piezoelectric (PE) materials is viewed as a modern and innovative technology.5) The first use of vibrating PE microfibers to transfer mechanical energy into chemical energy by water splitting was reported by Hoffmann et al.6) in 2010. Researchers have revealed an increasing curiosity in PE catalysis as a potential solution for environmental remediation in recent years. It has been revealed that PE catalysis uses mechanical vibration energy, a readily available resource that is prevalent in daily life,7) including walking8) and river flow.9) PE materials10,11) have the ability to accumulate vibrational energy and discharge significant quantities of free electrical charge, which subsequently undergo a chemical reaction with water. This particular process is referred to as PE catalysis. Electrical energy can be converted from mechanical vibrations employing the PE material’s potential field.12,13,14,15) It is essential to understand that, when exposed to the potential field, the free charge carriers produced during the PE catalytic reaction can easily be separated onto the surface of the catalyst.16,17) Therefore, PE catalysis, which offers a higher catalytic efficiency than photocatalysis, is a promising technique that could improve photogenerated charge separation.
Barium titanate, often known as BaTiO3 (BTO), is the earliest known ferroelectric ceramic to be discovered recently and has been used in numerous industrial applications due to its exceptional ferroelectric, piezoelectric, dielectric, and thermoelectric properties.18,19) Therefore, BaTiO3-based coatings have recently attracted increased interest from researchers.
Single-atom catalysis has been receiving significant attention in recent times since reducing the size of a nanoparticle to the atomic level can optimize the application of a noble metal by promoting its dispersion. Want et al.20) employed thermal treatment to facilitate the transportation of Ag nanoparticles (NPs) along an outer surface and their insertion into the channels of MnO2 nanorods with a hollandite structure. This led to efficient catalytic oxidation of HCHO due to the outstanding Ag particle dispersion at the sub-nanoscale level, including individual atoms. Catalyst decorating with finely distributed noble metals is still very difficult due to the tendency of Ag or Au NPs to agglomerate into large particles during the Ostwald ripening process, owing to their high surface energies. Furthermore, a great deal of noble-metal atoms is dispersed throughout the bulk during conventional synthesis processes like coprecipitation or photo deposition, rendering them ineffective as active sites. Several studies have suggested that the atomic metal may develop stronger interactions with MnO2 compared to an Ag cluster and MnO2. The result is an increase in catalytic activity due to better charge transfer in the catalyst.20,21)
This research paper presents the design and fabrication of a PE catalytic system employed in the hydrothermal and ultrasonic synthesis of a Cu-BaTiO3 composite structure with synergistic catalytic effects. Pharmaceutical pollutants may be effectively destroyed using this system in the presence of ultrasonic vibrations. An increased PE potential on the cataly stsurface induces movements in the conduction and valence bands, facilitating electron and hole migrations that are both more efficient and fast during the reaction with dissolved oxygen. Throughout four consecutive PE catalytic processes, the Cu-BaTiO3 nonmaterial showed outstanding stability and PE catalytic activity.
2. Experimental Procedure
2.1. Chemicals
The barium chloride dehydrate (BaCl2 ‧ 2H2O) was provided by Shanghai Silian Chemical Plant, while the tetrabutyl titanate (C4H9O)4 ‧ Ti was obtained from Jiangsu Qiangsheng Functional Chemical Co., Ltd. and the sodium hydroxide (NaOH) were acquired from Hubei Chemical Reagent. The oxygen was procured from Suzhou Jinhong Gas Co., Ltd. To produce BTO nanopowder in its cubic phase, the aforementioned chemicals were used as the precursors. Tetracycline was acquired from Aladdin, and ammonia (NH3 ‧ H2O) was procured from Wuxi Zhanwang Chemical Reagent Co., Ltd. All chemicals that were utilized were analytical grade reagents except for the pure (C4H9O)4 ‧ Ti solution. The reagents were applied exactly as supplied. Deionized (DI) milli-Q water with a resistivity of 18.2 MΩ ‧ cm was employed as the solvent for each of the prepared solutions.
2.2. Anchoring monoatomic Cu to BaTiO3
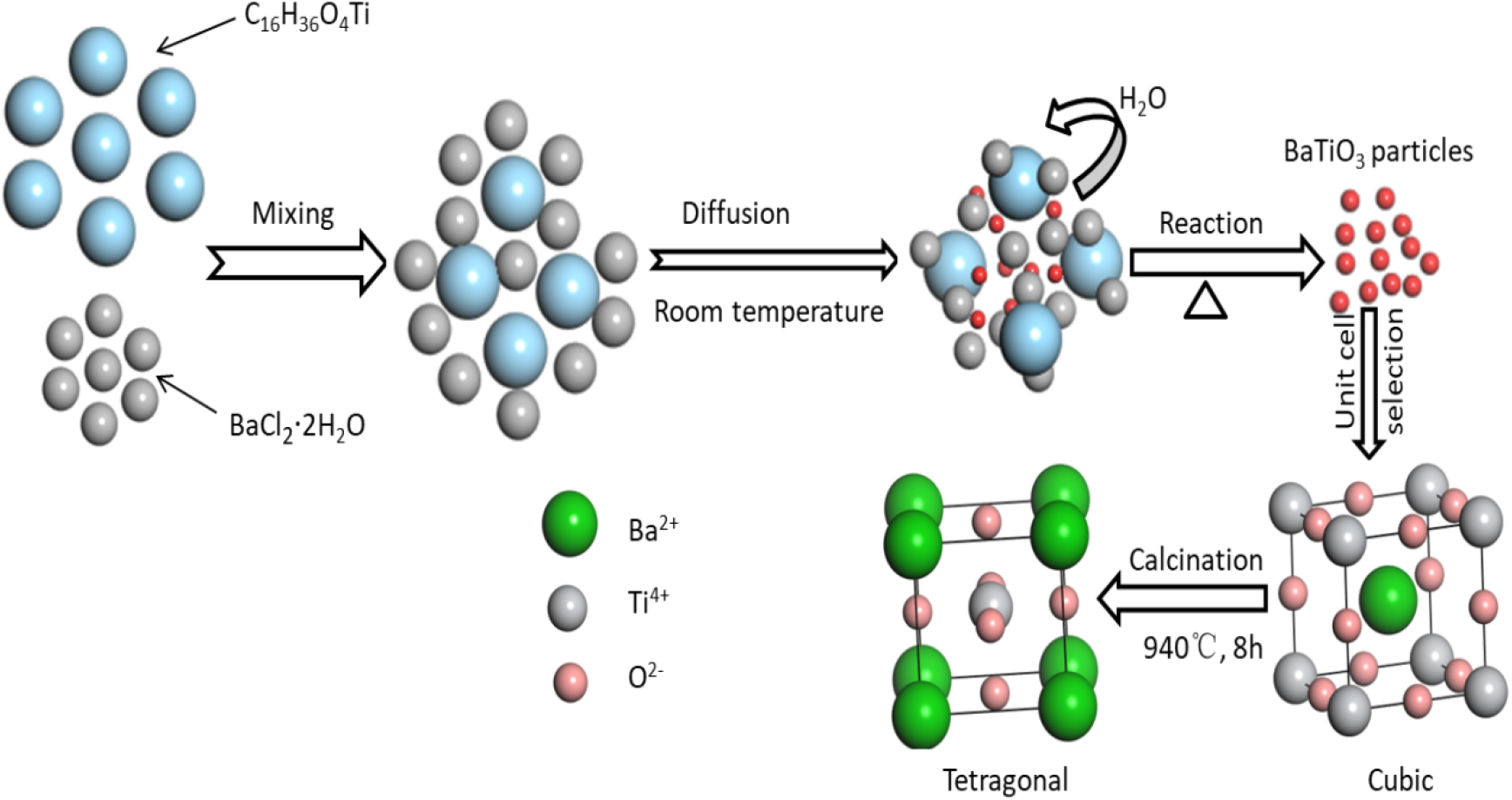
Fig. 1 illustrates the process of preparing the sample. The preparation of single-atom Cu-BaTiO3 was conducted in the following manner. BaCl2 ‧ 2H2O (7.5 mmol) was dissolved in 30 mL of DI water using ultrasonication in a 100 mL beaker. Then, (C4H9O)4 ‧ Ti (2 mL) was introduced dropwise while the mixture was stirred for 30 min to ensure thorough mixing. The dissolved substance was introduced dropwise into the solution while agitating continuously for 1 h to facilitate a complete reaction, after the ultrasonic dispersion of approximately 75 mmol of NaOH in DI water (30 mL). After the diluted mixture was added to a 100 mL hydrothermal autoclave and the mixture was heated to 180 °C for 16 h, vacuum filtration was performed on the reaction mixture once the product had cooled to room temperature. To obtain a cubic BaTiO3 sample, the obtained sample was rinsed several times with 100 % ethanol and DI water. It was then vacuum-dried for 12 h at 70 °C in an oven. Following sample collection, it was put into a 50 mL crucible and heated at a rate of 5 °C per min in a muffle furnace to 940 °C. The temperature then remained constant for 8 h. The product was then cooled to ambient temperature and the crucible comprising tetragonal BaTiO3 was taken out.
The second set of steps included employing the thermal equilibrium approach to attach the single Cu atom to the tetragonal-phase BTO. Following this, a volume of 50 mL of CuSO4 ‧ 5H2O was introduced into ammonia solutions with different concentrations (0.008, 0.01, 0.012, 0.014, 0.016, and 0.018 mol/L), leading to the formation of a blue turbidity. The addition of the solution was continued until the complete dissolution of the precipitate. Afterward, the solution was added with a particular amount of the calcined BaTiO3 while stirring for the duration of 1 h. The obtained solution was subsequently transferred into a 100 mL autoclave. The sample was rinsed and dried at 70 °C for 12 h in a vacuum oven following the process of cooling down the reaction till room temperature. The sample was then calcined at 200 °C in a muffle furnace for 2 h after being placed in a crucible. The obtained single-atom Cu-anchored BTO sample then underwent characterization and electro-Fenton catalytic degradation.
2.3. Characterization and PE activity test
High-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) was carried out on a THEMIS Z (FEI) apparatus that was outfitted with a cold field emission gun operating at 80 kV and double aberration correctors. The energy dispersive X-ray (EDX) mapping was performed at an applied voltage of 200 kV. An X'Pert-Pro MCU X-ray diffract to meter from Panalytical was employed to perform X-ray diffraction (XRD). At 40 mA current and 40 kV voltage, the X-ray source released Cu-Kα radiation with 0.154 nm wavelength. The catalyst’s surface chemical composition was examined utilizing X-ray photoelectron spectroscopy (XPS) with the sample being evaluated using a monochromatic Al-Ka source as the X-ray source. A high-resolution transmission electron microscope (HRTEM) was employed to investigate the microstructures of the BTO samples anchored with single-atom Cu. Consequently, tetracycline was used as the target pollutant to assess the PE catalyst’s degrading efficiency. A standard procedure involved the dispersion of 0.1 g of Cu-BaTiO3 in tetracycline solution (50 mL, 8 mg/L).Oxygen was added to the mixture for 20 min to combine the oxygen already present in the solution. The solution was subsequently agitated for 5 min to allow the catalyst to achieve adsorption equilibrium. The power rating and ultrasonic vibration frequency of the ultrasonic cleaning equipment were 100 W and 40 kHz, respectively. The tetracycline degradation kinetics were studied by collecting 3 mL of the solution at regular intervals and then separating it by centrifugation to get the clear liquid part. The UV-visible absorption spectra were then obtained and analyzed using a Beijing Puxi TU-1901 spectrophotometer to determine the concentration of tetracycline in the centrifugal fluid. Furthermore, recovery of the used catalyst (Cu-BaTiO3) in the experiment was carried out followed by re-exposure to catalytic degradation assays to assess the catalyst’s cyclic stability for tetracycline degradation.
3. Results and Discussion
3.1. XRD analysis
The XRD pattern of BaTiO3 loaded with monoatomic copper, both before and after calcination, is illustrated in Fig. 2(a-c). Curve c represents a typical cubic phase barium titanate (CUF#75-0212, space group: Pm-3m, a=b=c=4.016 Å, a=b=g=90°). The presence of distinct peaks at (100), (110), (111), (200), (211), and other sharp peaks indicates that the prepared sample is cubic BaTiO3 of the perovskite-type. The comparison of curves b and c revealed the bifurcation peak of curve b at 2q = 45.2°. After calcination, the BaTiO3 crystal phase changed from cubic to tetragonal (CUF#75-2121, space group: P4/mm, a=b=3.989 Å, c=4 Å, a=b=g=90°). A comparison of curves presented in a and brevealed that the planar structure of Cu-BaTiO3 remained the same after further calcination, maintaining its tetragonal phasestructure.22)
3.2. Observations of TEM and HAADF-STEM
The TEM images offer more insights into the field of crystallography. Fig. 3(a) demonstrates the successful crystallization of BaTiO3 NPs with a size of approximately 200 nm. A single crystal grain showed well-organized lattice fringes in the HRTEM image. The tetragonal BaTiO3 was measured to have a lattice fringe spacing of 0.28 nm, which corresponds to the value specified on the standard CUF card (JCCUS Card No. 79-2264) for the crystal plane fringe spacing (101).
Furthermore, HAADF-STEM and EDX technology were employed to investigate the shape and composition of Cu-BaTiO3 further. The distance between the (001) crystal planes (d001 = 0.402 nm) was found to be slightly greater than the (100) planes (d100 = 0.393 nm), as illustrated in Fig. 4, based on the electron diffraction pattern of the selected area [Fig. 4(b, c)]. The perovskite tetragonal symmetry was confirmed by the divergent behavior of the lattice parameters. The HAADF-STEM [Fig. 4(d)] and the associated elemental mapping images [Fig. 4(e-h)] demonstrated the uniform distribution of barium (Ba, represented in red), titanium (Ti, shown in green), oxygen (O, indicated in blue), and copper (Cu, represented in yellow) within the nanostructures. This appeared to show that single-atom Cu had been successfully added to BaTiO3. In the monoatomic Cu-BaTiO3, the atomic ratios of O, Ti, Ba, and Cu were 61.45 %, 24.31 %, 13.94 %, and 0.3 %, respectively. A reasonable atomic stoichiometric ratio was observed between the metal (Ba + Ti + Cu) and O, with a ratio of 2:3.18, which is close to 2:3.
3.3. XPS analysis
The XPS results reveal that Cu-BaTiO3 contains Ba 3d, Cu 2p, Ti 2p, and O 1s. Fig. 5(a-d) illustrate the HR-XPS spectra of the elements showing the consistency with the EDX findings displayed in Fig. 4(h). Fig. 5(a) displays the HR-XPS results of Ba 3d. The electron affinities of Ba 3d5/2 and satellite peak showed a distinctive peak at 778.4 and 780 eV, respectively. The distinctive peaks from Ti 2p may be found at 457.9 and 463.7 eV [Fig. 5(b)]. Fig. 5(c) of Cu 2p shows the respective Cu 2p1/2 and Cu 2p3/2 peaks at 952.6 and 932.8 eV. The absence of satellite peaks indicates that the sample comprises monovalent copper, Cu0, or a combination of both. The O 1s distinctive peaks were observed at 532.1 and 529.5 eV.23,24,25,26)
3.4. Piezoelectric characteristics of the sample
To ascertain the factors underlying the visible increase in the catalytic performance of the binary composite Cu-BaTiO3, transient PE current response experiments were conducted on BaTiO3 without and with pressures, as well as Cu-BaTiO3, using a computer-controlled electrochemical workstation (CHI660C). To validate the piezo-electric effect, the BaTiO3 and Cu-BaTiO3 piezo-currents were determined by applying a 1.1 ± 0.1 kg cm2 pressure between the ITO electrodes. Fig. 6 demonstrates that the PE current density of the binary composite Cu-BaTiO3 was notably greater than that of the single BaTiO3. This suggests that the binary composite possesses superior carrier separation performance and enhanced electron mobility, making it suitable for use as a highly effective PE synergistic active catalyst.
3.5. Tetracycline degradation
3.5.1. Degradation rate analysis
Thetetracycline degradation was examined to demonstrate the combined impacts of Cu-BaTiO3 composites on catalytic potential, as depicted in Fig. 6. To compare, the adsorption capacity of pure BaTiO3 and the catalytic capacities of pure BaTiO3 and Cu-BaTiO3 were evaluated using identical experimental conditions. Fig. 7 shows the adsorption capacity of pure BaTiO3 in dark settings [line (a)], the degradation of tetracycline by pure BaTiO3 during ultrasonic vibration [line (b)], and by the Cu-BaTiO3 composite [line (c)]. As expected, the tetracycline solution showed minimal degradation when exposed to pure BaTiO3, which only exhibited adsorption capability [Fig. 7(a)], or when subjected to ultrasonic vibration [Fig. 7(b)].
Fig. 7 shows that under dark conditions, pure BaTiO3 adsorbed around 30.1 % of the tetracycline. However, when subjected to ultrasonic vibration, the tetracycline degradation rate was enhanced to 56.2 % by the same material. The addition of Cu to the BaTiO3 composite resulted in a significant enhancement in the piezocatalytic oxidation activity, increasing it from 30.1 % to 81.3 %. Due to the combined effects of Cu and BaTiO3, the Cu-BaTiO3 composite exhibited superior degrading properties when subjected to ultrasonic vibration.
3.5.2. Effects of Cu content
Fig. 8 illustrates how samples with varying concentrations of Cu cause the tetracycline solution to degrade. Various concentrations of CuSO4 ‧ 5H2O solutions (0.008, 0.01, 0.012, 0.014, 0.016, and 0.018 M) were produced. The tetracycline degradation efficiencies were greatly affected by the concentration of Cu. The efficiency increased from 71.6 to 81.3 % following 7 h of ultrasonication when the dose of CuSO4 ‧ 5H2O raised from 0.008 to 0.012 mol/L. However, a reduction inefficiency was observed from 81.3 to 58.7 % under the same conditions with an increase in the dosage of CuSO4 ‧ 5H2O from 0.012 to 0.018 mol/L.
3.5.3. Cycle stability
Degradation experiments were conducted using the recovered Cu-BaTiO3 to assess the PE catalyst’s cycle stability (Fig. 9). The tetracycline degradation efficiency remained relatively unchanged during four cycles of degradation studies, suggesting that the Cu-BaTiO3 PE catalyst possesses excellent cycle stability.
3.6. Mechanism discussion
Due to the material’s non-centrosymmetric characteristics (i.e., crystal structure devoid of a symmetry center), electric dipoles developed within the substance, resulting in PE properties. The unit cell of PE catalyst was not symmetrical, unlike in other crystals. Although PE crystals have an asymmetrical arrangement of atoms in their lattice, they maintain electrical neutrality due to the cancellation of positive charges by nearby negative charges. The stress-induced compression or stretching of a PE crystal results in the displacement of its constituent atoms from their initial positions. The aforementioned displacement induces a general electrical charge to accumulate on the crystal, leading to the development of positive and negative charges on opposing outer surfaces.27,28) Applying stress to the unit cell caused a displacement of the initial atomic positions of the Ba2+ cations and the O2− anions, leading to the dipole moment formation within the unit cell. Therefore, the crystal obtained a PE potential, referred to as the piezopotential, which was generated by the combined polarization of charges in each cell within the crystal. Mechanical stress stimulates the PE crystal, resulting in the formation of negative as well as positive potentials on the opposite polarized crystal surfaces. These potentials are referred to as positive polarization domains (PCUs) and negative polarization domains (NCUs), respectively. The carriers can be separated for catalytic degradation using the electric field that develops within the crystal. The formation of free charges along with their associated role in the piezocatalytic redox reactions are demonstrated in reactions Eq. (1), (2), (3), (4), (5), (6), (7), (8), (9), (10), (11).
Anode (negatively charged BaTiO3 surfaces):
Overall:
Cathode (positively charged BaTiO3 sides):
Overall:
Tetracycline decomposition:
As a result, a schematic design was suggested to show how the charge carrier transport processes changed when Cu was added to the BaTiO3 crystal face. Fig. 10 displays schematic representations illustrating the charge carrier transport as well as separation processes occurring at the interfaces of the Cu-BaTiO3 composites. When ultrasonic vibrations were applied to the Cu-BaTiO3 system, the primary cell of BaTiO3 expanded in the XYZ direction, resulting in the formation of a 2 × 2 × 2 supercell. The application of an external force on BaTiO3 resulted in the propagation of a nonzero dipole moment. Furthermore, the c-axis-oriented BaTiO3 showed an internal electric field throughout its cross section as a consequence of the positive and negative piezo potentials on the compressed and stretched side surfaces, respectively. The electric field in the crystal is generated by the immobile and non-annihilating ionic charges. Being static and immobile, the polarization charges remain within the crystal throughout the entire duration of the stress.29,30) Under compressive strain, the PCU attracts compensatory electrons toward the surface in the PE field, thereby reducing the bandgap, while the NCU exerts the repulsion of electrons from the surface, thereby providing an increase in the bandgap.
Cu was selectively deposited onto the BaTiO3 crystal face to further demonstrate the critical consequences of the redistribution of charge density inside the NPs. The formation of diffusion channels of charge carriers at the Cu-BaTiO3 interfaces was facilitated by the numerous piezo-induced electrons escaping to the interfaces of Cu-doped BaTiO3, as illustrated in Fig. 10. This is due to the exceptional conductivity of Cu. Therefore, this mechanism inhibited charge recombination induced by the piezo and ultimately enhanced the activity of piezocatalytic oxidation.
4. Conclusion
In conclusion, monoatomic clusters Cu NPs anchored onto tetragonal BaTiO3 were developed in this study, and associated PE catalytic potential was investigated via degradation of tetracycline solution. The nanomaterial decomposed tetracycline at a rate of 95 % within 7 h when subjected to mechanical vibrations; which was significantly higher compared with the degradation rate observed for pure BaTiO3 (56.7 %). Single-atom clusters of Cu facilitated the piezo-induced electron separation, thereby enabling the achievement of synergistic catalysis, due to the abundance of piezo-induced electrons that leak out to the Cu-doped BaTiO3 interfaces because of Cu’s excellent conductivity. The synthesized Cu-BaTiO3 NPs demonstrate remarkable stability and performance in four distinct PE catalytic processes.