1. Introduction
Energy is essential in modern society, and research on energy storage devices is gradually increased in the requirement of energy. Energy storage devices such as batteries and supercapacitors have been used to satisfy the demand for electric vehicles, various electronic devices and supplies.1,2,3,4,5,6) Supercapacitors, also called electrochemical capacitors, have outstanding electrochemical performance including high power density, fast charge/discharge kinetics, stable cycle performance and large specific capacitance etc.7,8,9) Furthermore, supercapacitors comprise electrodes, electrolyte, separator and current collectors, which might relate to the electrochemical performance. Transition metal oxide-based electrode materials for supercapacitors, such as ruthenium oxide (RuO2), manganese oxide (MnO2), cobalt oxide (Co3O4), nickel oxide (NiO), copper oxide (CuO), molybdenum oxide (MoO3), ferric oxide (Fe2O3) and vanadium oxide (V2O5), exhibit excellent electrochemical performance for attractive supercapacitive behaviors as high theoretical capacitance and high cycling stability.10,11,12,13,14,15,16,17)
Among transition metal oxide-based electrode materials, vanadium oxide-based materials (V2O5, VO2, V2O3, V6O13 etc.) has been frequently investigated as electrodes of energy storage devices for several years and has mostly synthesized by adding acid to vanadium precursor.17,18,19,20) Above all, the advantages of vanadium pentoxide (V2O5) are a layered structure, high theoretical specific capacitance, multiple oxidation valence state, low cost and natural abundance.21,22) In addition, hydrated vanadium pentoxide (V2O5 ‧ nH2O) that H2O inserted into V2O5 is expanded interlayer spacing of V2O5 material to improve electrochemical performance due to the expanded layered structure.23) However, V2O5 ‧ nH2O and V2O5 have low electrical conductivity and structural instability which might restrict implementation of energy storage devices.24,25,26) To solve these problems, V2O5 ‧ nH2O has been used along with carbon-based materials that substantial surface area and high electrical conductivity such as carbon nanotube (CNT), reduced graphene oxide (rGO) and graphene oxide (GO).27,28,29) Geng and Wang28) developed V2O5 ‧ nH2O/graphene composite for supercapacitors that was greater electrochemical performance than V2O5 ‧ nH2O xerogel by adding GO. Fan et al.29) reported V2O5 ‧ nH2O/rGO composite film that V2O5 ‧ nH2O was sandwiched with rGO by chemical bonding to ensure good mechanical properties, which exhibited high specific capacitance and good cycling stability for ammonium-ion energy storage. In particular, abundant oxygen functional groups and high specific surface area of GO increase electrochemical performance.30,31) Dai et al.30) developed MnO2 nanowires/GO nanosheets composite that possessed high specific capacitance and high cycle performance due to the oxygen functional groups and high specific surface area of GO, and the composite was prevented aggregation of GO and MnO2 nanowires and accordingly provided high specific surface area. Additionally, electrochemical performance might vary according to various drying processes at pressure and temperature.32) Azadvari et al.32) reported MXene of a three-layered structure that had better electrochemical performance and higher capacitance retention under vacuum-drying condition at 80 °C than under air-drying condition at 25 °C.
Herein, we demonstrate that increase electrochemical performance of V2O5 ‧ nH2O/GO nanobelts added with GO through a simple microwave-assisted hydrothermal synthesis. Moreover, we manufacture V2O5 ‧ nH2O/GO nanobelts based on water without using acid. V2O5 ‧ nH2O/GO nanobelts exhibit high specific capacitance of 206 F/g at 0.1 A/g and enhanced cycle stability, which is better than V2O5 ‧ nH2O that shows specific capacitance of 108 F/g at 0.1 A/g and low cycle stability. Also, we investigate differences in electrochemical performance of V2O5 ‧ nH2O/GO nanobelts depending on drying processes of carbon paper electrode at different pressure and temperature. These electrochemical properties of V2O5 ‧ nH2O/GO nanobelts become a potential candidate as an electrode for supercapacitors.
2. Experimental Procedure
V2O5 ‧ nH2O/GO, the result of V2O5 and GO in water, was manufactured by mixing V2O5 powder (Sigma-Aldrich, item No. 223794), GO (CHARMGRAPHENE, 2 mg/mL), and DI water and then using a microwave-assisted hydrothermal method. 7 mg V2O5 was mixed in 15 mL water and stirred for 12 h using a magnetic bar, then stirred for 3 h at 50 °C. This solution was filtered using PVDF filter paper with a pore size of 0.22 µm. The filtered solution of 12 mL was added GO 0.1 mL and sonicated for 10 min. This solution was transferred to glass vial and reacted microwave-assisted hydrothermal process with stirring at 150 °C for 1 h. The product was vacuum filtered using the filter paper and washed with water. Additionally, V2O5 ‧ nH2O was prepared in the same process by excluding GO.
A mixture of completely reduced VO2 and partially reduced V10O24 ‧ nH2O, a result of V2O5 in oxalic acid (Sigma-Aldrich, item No. 194131), was manufactured by mixing V2O5, water, and oxalic acid and then using a microwave-assisted hydrothermal method. 0.727 g V2O5 and 1.080 g oxalic acid were mixed in 72 mL water and stirred for 12 h using a magnetic bar. This solution was transferred to glass vial and reacted microwave-assisted hydrothermal process with stirring at 150 °C for 1 h.
The product was vacuum filtered using the filter paper and washed several times with water and ethanol.
The structural characterization of the results created by the above process was measured. The X-ray diffraction (XRD) patterns were obtained by an X-ray diffractometer (Rigaku, MiniFlex 600) using Cu-Kα radiation in the range of 3° to 60° (2θ) with scanning rate of 2°/min. The Raman spectra were measured by a Raman spectrometer (Thermo Fisher Scientific, DXR3xi) with laser excitation of 532 nm at power of 1 mW. The morphology was evaluated by a field emission scanning electron microscopy (FE-SEM; Tescan, CZ/MIRA I LMH).
A process was carried out to measure electrochemical properties as follows. 1 mg active material, 20 µL nafion, 200 µL ethanol and 800 µL water were mixed using ultrasonicator for 20 min. This solution of 30 µL was dropped on carbon paper and dried at different processes then the dried carbon papers were used the electrode. A drying process of the carbon paper, low-vacuum (500~760 torr) room temperature (RT) process, the carbon paper was dried in oven at 60 °C for 2 h and in low vacuum at RT for 12 h. Another drying process of the carbon paper, high-vacuum (10-3~10-4 torr) 100 °C process, the carbon paper was dried in oven at 60 °C for 15 min and then dried in high vacuum at 100 °C for 2 h.
The electrochemical measurement was assessed by a three-electrode system using electrochemical workstation (Ivium Technologies, IviumStat.h). an Ag/AgCl electrode, a graphite rod and the active material on carbon paper were used as the reference electrode, the counter electrode and the working electrode. The electrolyte was used by 2 M KCl solution. The cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrochemical impedance spectroscopy (EIS) were executed. The voltage window for CV and GCD measurements was 0 to 0.8 V. The frequency range for EIS was from 100 kHz to 0.1 Hz with an amplitude of 5 mV. The specific capacitance (CS, F/g) was calculated from CV curve and GCD curve by the following Eqs. (1) and (2).33,34)
where, is the integrated area of the CV curve, (g) is the mass of active material on carbon paper, (mV/s) is the scan rate, (V) is the potential window.
where, (A) is the discharge current, (s) is the discharge time, (g) is the mass of active material on carbon paper, (V) is the potential change during discharge process.
3. Results and Discussion
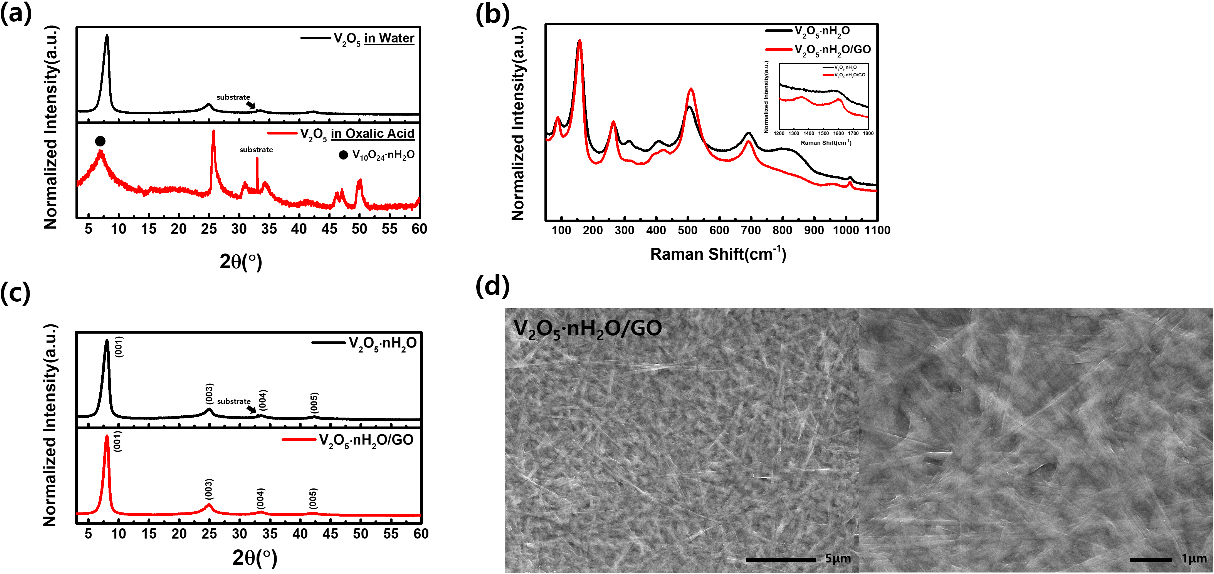
The synthesis process of V2O5 ‧ nH2O/GO nanobelts is shown in Fig. 1. V2O5 powder was dissolved in deionized water and filtered to make V2O5 solution with uniformly diffused V5+ ions. The V2O5 solution was mixed with GO and sonicated that completely dispersed. This solution formed V2O5 ‧ nH2O/GO through microwave-assisted hydrothermal reaction. The result became a flexible paper using vacuum filtration. Vanadium oxide-materials including V2O5 ‧ nH2O were chiefly generated by adding acids (hydrogen peroxide, oxalic acid, citric acid, sulfuric acid etc.) to vanadium precursor.35,36,37,38,39,40,41) The resultant existed as a mixture in Fig. 1, VO2 and V10O24 ‧ nH2O, when oxalic acid was added to V2O5 powder and water. However, inset digital image (Fig. 1) and Fig. S1 show that GO cannot be dispersed in oxalic acid (pH 1). Because of the relationship between GO and pH, it was not completely dispersed in acid (pH 1), therefore it was likely that the effect of GO would not be applied uniformly.42)

Fig. 1.
Illustration on the preparation of V2O5 ‧ nH2O/GO nanobelts. Image (left) of black dotted box is a mixture of completely reduced VO2 and partially reduced V10O24 ‧ nH2O after microwave-assisted hydrothermal reaction process of V2O5 in oxalic acid. Image (right) of black dotted box is not uniform diffusion of GO when V2O5 + GO in oxalic acid. In image of red dotted box, V5+ ions and GO are uniformly mixed by sonication and formed V2O5 ‧ nH2O/GO nanobelts after microwave-assisted hydrothermal reaction process of V2O5 + GO in water.
Fig. 2(a) shows characteristic diffraction peaks that V2O5 has different results in water and oxalic acid after microwave-assisted hydrothermal reaction. Si/SiO2 substrate was used to support the results and the substrate peak is observed in the XRD pattern. The XRD pattern of V2O5 in oxalic acid shows as the mixture that exists completely reduced VO2 and partially reduced V10O24 ‧ nH2O. VO2 is completely reduced from V5+ to V4+. The reduction reaction of VO2 using V2O5 and oxalic acid is described by following Eqs. (3) and (4).43)
However, due to a low temperature process condition of 150 °C or soft-reducing condition, V5+ is partially reduced to produce V10O24 ‧ nH2O.44,45) The XRD pattern confirms that is included V10O24 ‧ nH2O (JCPDS No. 00-025-1006) among the peaks of VO2 (JCPDS No. 01-084-3056). The XRD pattern of V2O5 in water shows which is not reduced from V5+. Compared with XRD analysis of V2O5 powder (Fig. S2), H2O is inserted into V2O5 to become V2O5 ‧ nH2O (JCPDS No. 00-040-1296) after microwave-assisted hydrothermal reaction. Fig. 2(b) displays Raman analysis to identify structures of vanadium oxide. V2O5 ‧ nH2O has vibration modes of chemical bond related to vanadium and oxide in 50~1,100 cm-1. The bands at 89 cm-1 and 156 cm-1 in low-frequency mode are assigned to the lattice vibration with layered structure.46,47) The V=O bending mode is observed at 266 cm-1 and 407 cm-1.48) The bands at 316 cm-1 corresponds to the V3-O bending modes.49) The band at 503 cm-1 is assigned to the stretching mode of the V3-O mode.48) The stretching mode of V2-O is observed at 692 cm-1.46) The stretching mode of V=O is located at 1,012 cm-1.48) During microwave-assisted hydrothermal reaction, water molecules are inserted between V2O5 layers to intense peak of 503 cm-1 and 692 cm-1.50) The Raman analysis of V2O5 ‧ nH2O/GO also shows chemical bonds related to V-O type and is similar to V2O5 ‧ nH2O in 50~1,100 cm-1. In the inset image of Fig. 2(b), V2O5 ‧ nH2O/GO has D peak and G peak that demonstrate the existence of carbon materials in 1,200~1,800 cm-1. The G peak at 1,600 cm-1, representing the E2g mode, is shifted due to oxygenation.51) In addition, the reduction in size of in plane sp2 domains in graphite after oxidation, resulting in a broadened D peak at 1,353 cm-1.52)Fig. 2(c) shows V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O which correspond to (001), (003), (004), (005) lattice plane. These planes confirm a layered structure of V2O5 ‧ nH2O (JCPDS No. 00-040-1296). The characteristic peaks of V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O comprise similar peak positions, which means that GO not affect the lattice position. Si/SiO2 substrate peak is observed in the XRD pattern. As displayed in Fig. 2(d) and Fig. S3, V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O exhibit a nanobelt-like morphology. However, unlike V2O5 ‧ nH2O in Fig. S3, V2O5 ‧ nH2O/GO in Fig. 2(d) shows a clear nanobelt morphology due to likely prevented aggregation of GO and V2O5 ‧ nH2O.30,31)
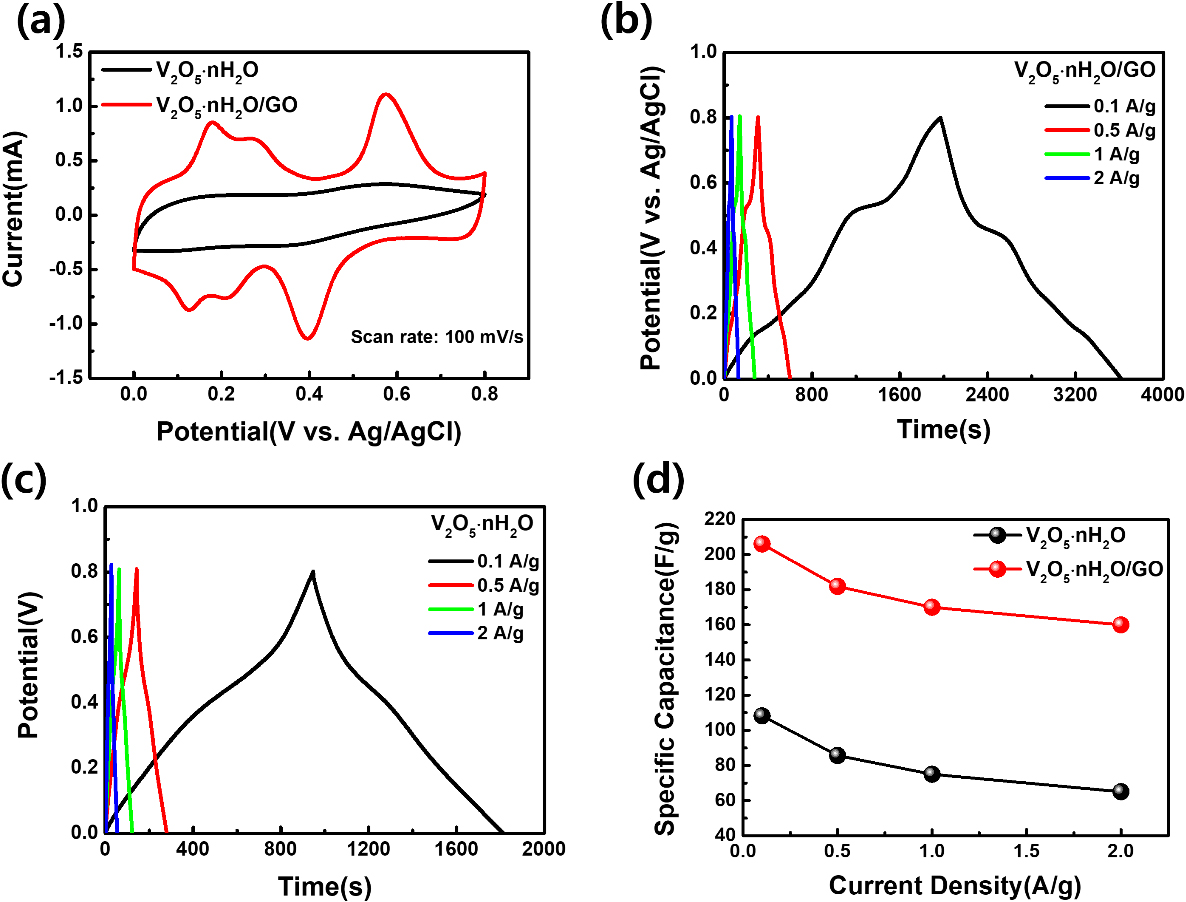
Fig. 3(a) displays the CV curves of V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O between 0 and 0.8 V at a scan rate of 100 mV/s. Fig. 3(a) shows the scan rate of 100 mV/s among the CV curves at different scan rates which measured in order from 5 mV/s to 100 mV/s in Fig. S4(a, b). When comparing the integrated area under CV curves, specific capacitance is larger in V2O5 ‧ nH2O/GO. Therefore, the calculated specific capacitance of V2O5 ‧ nH2O and V2O5 ‧ nH2O/GO at a scan rate of 100 mV/s is 127.58 F/g and 350.86 F/g. Fig. 3(b, c) indicate the GCD curves at various current densities of V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O. As shown in Fig. 3(d), the GCD curves at various current densities of V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O are manifested as the calculated specific capacitance. The specific capacitance of V2O5 ‧ nH2O/GO is 206, 182, 170, 160 F/g under current density of 0.1, 0.5, 1, 2 A/g. The specific capacitance of V2O5 ‧ nH2O is 108, 86, 75, 65 F/g under current density of 0.1, 0.5, 1, 2 A/g. Accordingly, V2O5 ‧ nH2O/GO under the same measuring conditions has greater specific capacitance than V2O5 ‧ nH2O due to abundant oxygen functional groups and high specific surface area of GO.30,31)
The Nyquist plots of V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O from 100 kHz to 0.1 Hz frequency region are presented in Fig. 4(a) and based on the equivalent circuit of Fig. S5. V2O5 ‧ nH2O/GO in Fig. 4(a) shows larger slope of the sloping line in low frequency region than V2O5 ‧ nH2O that indicates the fast diffusion rate of ions.53) Moreover, Fig. 4(b) shows the Nyquist plots at high frequency region. The diameter of semicircle in Fig. 4(b) implies charge transfer resistance (Rct), the electrode resistance, which is associated to the surface area and conductivity of the electrode.54) The Rct values of V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O are 6.0 Ω and 20.2 Ω. The low resistance value of V2O5 ‧ nH2O/GO is due to the inclusion of GO that features high conductivity and high surface area.55) Additionally, the low resistance value of V2O5 ‧ nH2O/GO exhibits a fast electrochemical reaction.56)Fig. 4(c) shows the cycling performances of V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O during 200 cycles and 100 cycles at scan rate of 100 mV/s. Cycling performance of V2O5 ‧ nH2O has specific capacitance retention rate of 57.26 % in 100 cycles. Cycling performance of V2O5 ‧ nH2O/GO has specific capacitance retention rate of 85.91 % in 100 cycles and 73.84 % in 200 cycles. V2O5 ‧ nH2O/GO indicates better cycling performance than V2O5 ‧ nH2O, which is due to GO possesses high specific surface area and abundant oxygen functional group.30,31)Fig. 4(d) shows cycling performances depending on the drying processes. The high-vacuum 100 °C process and the low-vacuum RT process have capacitance retention of 81.65 % and 73.84 % at 200 cycles. The high-vacuum 100 °C process has better cycling performance than the low-vacuum RT process due to the drying process at high vacuum and high temperature.32) In addition, Fig. S6, the initial cycle curves of cycling performance in Fig. 4(d) at different drying processes, has specific capacitance of 388.4 F/g in the high-vacuum 100 °C process and the specific capacitance (388.4 F/g) greater than specific capacitance of 385.08 F/g in the low-vacuum RT process.
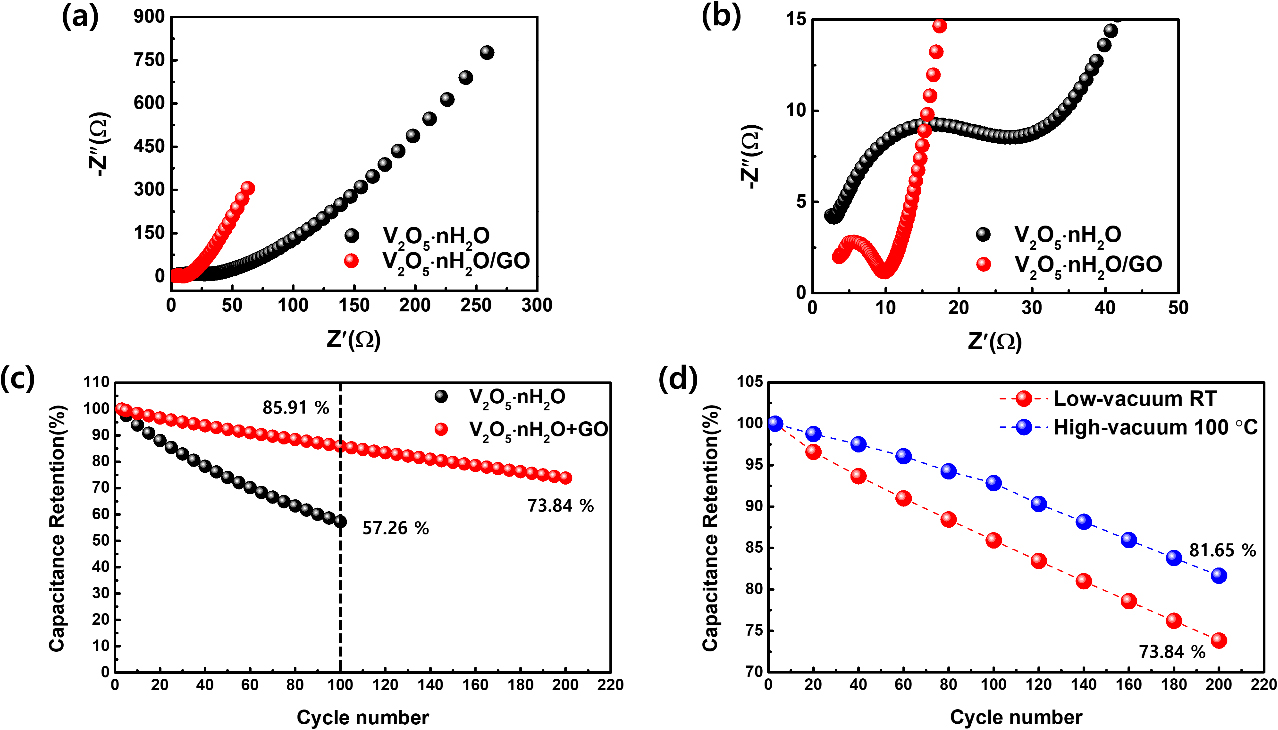
Fig. 4.
(a) Nyquist plots of V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O from 0.1 Hz to 100 MHz region. (b) Nyquist plots of V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O in high-frequency region. (c) Cycling performances of V2O5 ‧ nH2O/GO and V2O5 ‧ nH2O during 200 cycles at scan rate of 100 mV/s. (d) Cycling performances of V2O5 ‧ nH2O/GO at different vacuum and temperature conditions in scan rate of 100 mV/s.
4. Conclusion
V2O5 ‧ nH2O/GO nanobelts were fabricated through microwave-assisted hydrothermal reaction by utilizing V2O5, water and GO. The structural properties of V2O5 ‧ nH2O/GO nanobelts that produced after the reaction were confirmed through XRD analysis, Raman analysis and SEM image. The electrochemical properties of V2O5 ‧ nH2O/GO nanobelts present high specific capacitance of 206 F/g at 0.1 A/g and stable cycling performance. V2O5 ‧ nH2O/GO nanobelts exhibit higher specific capacitance, cycling performance, lower charge transfer resistance and faster ion diffusion rate than V2O5 ‧ nH2O. In addition to using GO, V2O5 ‧ nH2O/GO nanobelts indicate great specific capacitance and cycling performance by drying at high vacuum and high temperature. These good electrochemical performances of V2O5 ‧ nH2O/GO nanobelts exhibit a great potential as an energy storage device electrode.