1. Introduction
2. Experimental Procedure
2.1. Synthesis of ZIF-67 on nikel foam (NF)
2.2. Synthesis of FeCo-LDH
2.3. Characterizations
2.4. Electrochemical measurements
3. Results and Discussion
4. Conclusion
1. Introduction
Electrochemical water splitting is a practical way to produce hydrogen (H2) because of its non-polluting, and renewable advantages.1,2) Oxygen evolution reaction (OER) leads to an increase in the energy required for hydrogen production due to the slow kinetic rate.3,4) Currently, the best OER electrocatalysts are made from noble metals such as iridium (Ir), and ruthenium (Ru) and their oxides. However, their scarcity and higher price hinder their commercial applications.5) Therefore, it is highly essential to develop earth-abundant, low-cost, durable, and efficient OER electrocatalysts.
Recently, layered double hydroxides (LDH) have emerged as the most promising candidates for OER catalysts due to their compositional and morphological flexibility.6) Among them, the low cost, straightforward synthesis, and adjustability of iron-cobalt layered double hydroxides (FeCo-LDH) have made them an excellent material for OER catalyst.7) However, stacking nanosheets in the LDH limited its catalytic site exposure and hence the performance.8) To address this issue, many studies have revealed that LDH synthesized using metal-organic frameworks (MOFs) as precursors and templates show great potential in water oxidation through morphology engineering and structure optimization.9,10) For example, Jiang et al.11) prepared three LDH hollow nanostructures using zeolitic imidazolate framework-67 (ZIF-67) as the template, and also demonstrated the influence of the type of metal (cation) in the salts on the morphology of LDH. Liu et al.12) transformed ZIF to prepare three types of CoMn-LDH through different manganese salts to understand the influence of anions on the crystal structure of LDH. Unfortunately, although the anionic groups of these metal salts can affect the structure and properties of MOF-derived materials, they remain understudied in addressing electrocatalytic hydrogen production, especially in low-dimensional nanomaterials.
Herein, we have demonstrated the fabrication of FeCo-LDH catalysts by reacting ZIF-67 with different iron salts [FeSO4 ‧ 7H2O, FeCl2 ‧ 4H2O, and Fe(NO3)3 ‧ 9H2O] and their applications in the OER. The FeCo-LDH-SO4 catalyst exhibits excellent electrochemical performance, realizing a low overpotential of 247 mV for the OER at 10 mA cm-2. Additionally, it demonstrates excellent durability for 40 h at 10 mA cm-2 for OER. According to experimental observations, the excellent OER activity of FeCo-LDH-SO4 is attributed to its unique shape, which can provide abundant gas release channels and accelerate mass transfer. In addition, its mixed structure can prevent the collapse and aggregation of nanostructures, which is beneficial to enhancing its OER activity and stability. Thus, this work provides a new way to create excellent OER electrocatalysts.
2. Experimental Procedure
2.1. Synthesis of ZIF-67 on nikel foam (NF)
Cobalt nitrate hexahydrate [Co(NO3)2 ‧ 6H2O : 0.5 mmol, 0.1455 g] and 2-methylimisdazole (C4H6N2 : 4 mmol, 0.3284 g) were dissolved in 10 mL deionized water (DI water) to prepare two separate solutions. A 4 × 1 cm2 NF was kept for 30 min in cobalt solution and then another solution was poured into the NF containing cobalt solution with continuous stirring for 2 h. After the reaction, NF was washed with DI water and dried at 60 °C. The deposited sample is denoted as ZIF-67.
2.2. Synthesis of FeCo-LDH
Three different iron salts [FeSO4 ‧ 7H2O, FeCl2 ‧ 4H2O, and Fe(NO3)3 ‧ 9H2O] are dissolved in 12 mL DI water and stirred for 10 min. The above synthesized ZIF-67 was immersed in respective iron salt solutions and stirred at 70 °C for 1 h to form FeCo-LDH, samples codes are given like FeCo-LDH-SO4, FeCo-LDH-Cl, and FeCo-LDH-NO3, respectively. Deposited samples are washed with DI water and then dried at 60 °C.
2.3. Characterizations
The surface morphology, composition and crystal structures of the synthesized samples were analysed by scanning electron microscopy (FE-SEM) with an energy dispersive X-ray detector (EDX), Powder X-ray diffraction (XRD), Raman, Fourier transform infrared (FT-IR), and X-ray photoelectron spectroscopy (XPS).
2.4. Electrochemical measurements
Using a three-electro system, electrochemical measurements were made with Autolab potentiostat (CHI Instruments, USA). The as-prepared electrocatalysts on NF served as the working electrode. The Ag/AgCl served as the reference electrode, Pt was used as the counter electrode and 1 M KOH was the electrolyte. The linear sweep voltammetry (LSV) was performed at a scanning rate of 1 mv s-1. The electrochemical impedance spectroscopy (EIS) was conducted using a high-performance potentiostat (Zive Potentiostat/Galvanostat/EIS, Wonatech, Korea) in 600 mV bias potential and a frequency range of 20,000 to 0.1 Hz. Electrochemical active surface areas (ECSAs) were calculated based on the formula: ECSA = Cdl/Cs. The electric double-layer capacitance (Cdl) was obtained by cyclic voltammetry (CV), while for Ni-/Co-based catalysts, specific capacitance (Cs) is usually adopted at 0.04 mF cm-2. The CV was tested in a range of 1.02 to 1.12 V vs. reversible hydrogen electrode (RHE) at different scan rates (10 to 50 mv s-1). The stability was tested at the current density of 10 mA cm-2 by chronoamperometry. All the potential values were converted to RHE by following equation: (RHER = EAg/AgCl + 0.059 PH + 0.197).
3. Results and Discussion
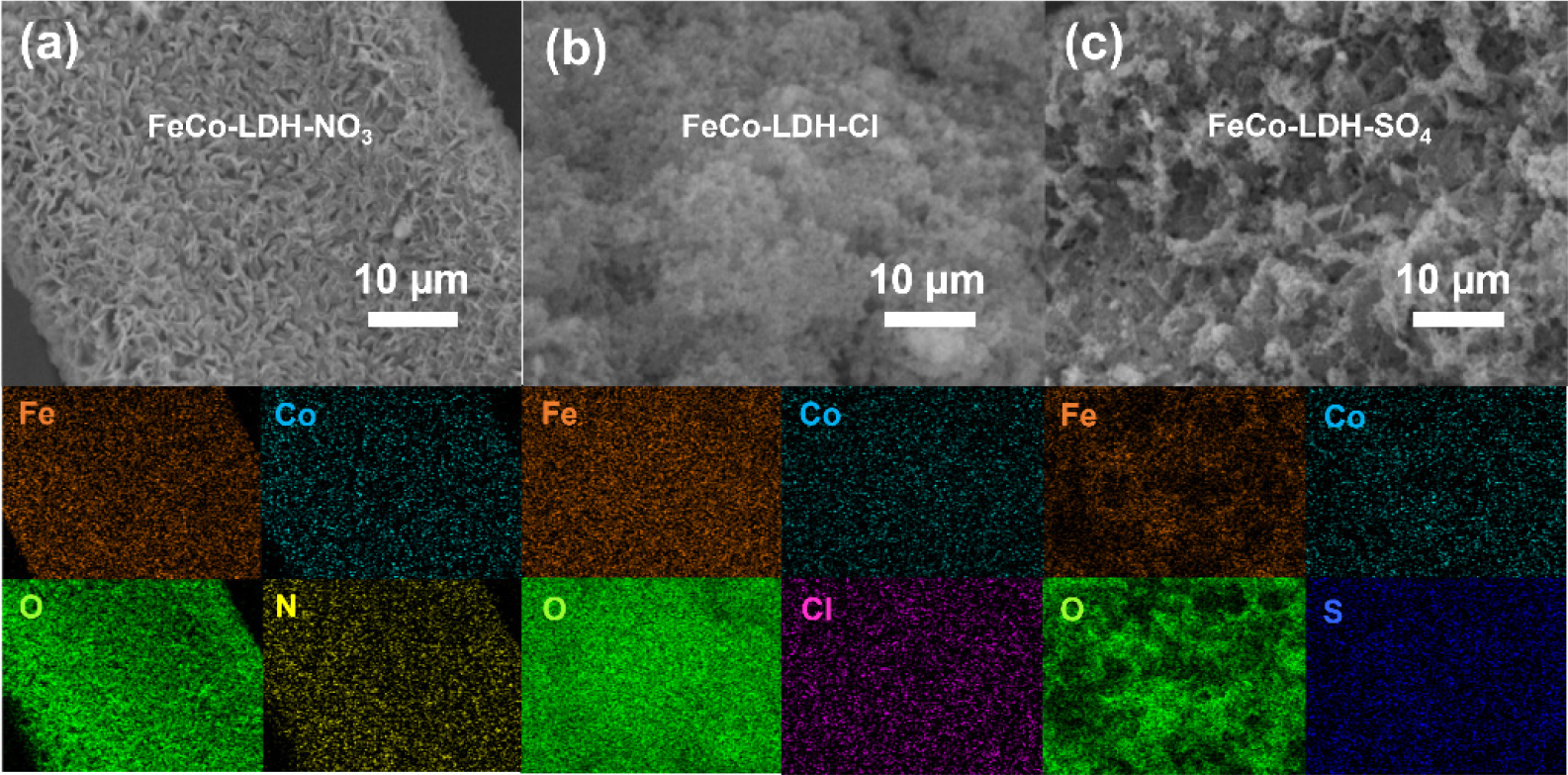
The XRD pattern in Fig. 1(a) shows successful synthesis of ZIF-67 which is consistent with the literature.13) The obvious LDH peaks were not observed in Fig. 1(b) due to the low crystallinity of the material and strong NF signals. FE-SEM image revealed that ZIF-67 has a nano triangular morphology [Fig. 1(c)]. The growth of FeCo-LDH was induced in different salt solutions at 70 °C by using nanotriangle-like ZIF-67 as a precursor. During the synthesis step, the ZIF-67 was quickly etched to release cobalt ions (Co2+) and 2-methylimidazole (2-MIM). Meanwhile, the dissociated Co2+ rapidly precipitated together with iron ions, hydroxyl ion (OH-) and carbonate (CO32-), which finally produced stable FeCo-LDH nanostructures. The resultant LDHs possess different morphologies due to the use of different anion salt solutions as reactants [Fig. 1(d-f)]. FeCo-LDH-SO4 showed a mixed structure of nanosheets and nanowires. FeCo-LDH-Cl exhibited a nano triangular structure compound of nanoparticles, and FeCo-LDH-NO3 displayed nanoparticle morphology with small particle sizes. The observed morphological differences are likely related to the solubility and rate of hydrolysis of the iron salts.12) The changes in nucleation and growth rates can describe the changes observed in the particle size of synthetic materials. The degree of dissociation of nitrate in aqueous solution is higher than that of sulfate. A higher activity can promote homogeneous nucleation and thus generate additional small nuclei. In the presence of sulfate, LDH is gradually grows. Enough time is allowed for maximum particle growth, resulting in the coexistence of small nanowires and larger nanosheets. In contrast, particles nucleate more quickly in nitrate and chloride salt environments, forming smaller particles or agglomerates.14)
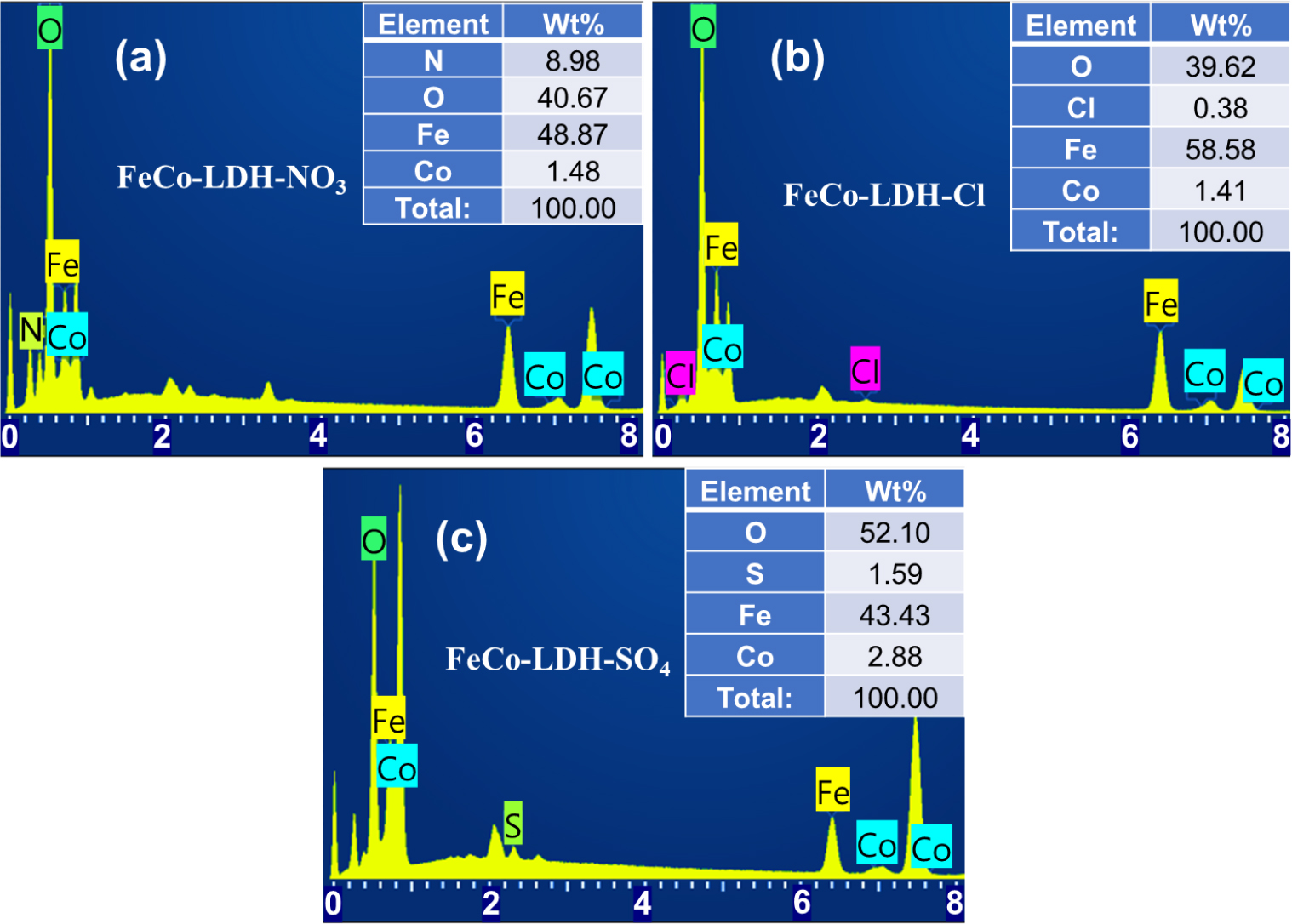
To confirm the impact of different anion salts on the element distribution and atomic ratio of the material surface, the EDX analysis of the samples produced by different iron salts is given (Fig. 2). From Fig. 2(a-c), it can be seen that the distribution of Fe, Co, and O in all samples is uniform, and the content of iron is much higher than that of cobalt. In addition, N, Cl and S were present in the corresponding samples, respectively, with the atomic ratio of this intercalating anion being between 1.4~1.7 at% (Fig. 3). Through EDS analysis, it can also be observed that the atomic ratios of Fe and Co in the three samples of FeCo-LDH-NO3, FeCo-LDH-Cl and FeCo-LDH-SO4, and are about 33:1, 41:1, and 15:1, respectively.
Raman spectroscopy further employed to study the vibration modes of the corresponding FeCo-LDH samples synthesized from different iron salt precursors. The peaks at 480 and 678 cm-1 were identified as Co-O and Fe-O bonding, respectively [Fig. 4(a)]. In addition, a new peak appeared at 549 cm-1, indicating the presence of a high oxidation state CoOOH in FeCo-LDH-SO4.15) Therefore, CoOOH was probably the real active site for the OER process. Studies have shown that the catalytic activity of electrocatalysts is improved by activating O ions with high-valent metal ions and promoting the adsorption of OER intermediates.16,17) The FT-IR spectra of the samples are shown in Fig. 4(b). These spectra exhibit bands near 3,350 and 1,630 cm-1, corresponding to the O-H vibrations of interlayer water molecules and the deformation vibrations of water molecules. FeCo-LDH-NO3 shows a peak at 1,333 cm-1 due to the presence of nitrate, while FeCo-LDH-SO4 and FeCo-LDH-Cl show two peaks at 1,467 and 1,346 cm-1 because of the carbonyl group stretching vibrations, arising from the dissolved CO2 in the DI water. As shown for FeCo-LDH-SO4, a single strong absorption related to SO4 occurs at 1,090 cm-1 with a shoulder at 1,018 cm-1.18)
Furthermore, the XPS survey spectrum of FeCo-LDH-SO4 showed the presence of Fe, Co, O, and S elements [Fig. 5(a)]. The two distinct peaks in the Fe 2p spectrum [Fig. 5(b)] are corresponds to the Fe 2p3/2 and Fe 2p1/2, respectively. Further Fe 2p3/2 and Fe 2p1/2 peaks deconvoluted into 710.4, 712.7, 724.2, and 726.2 eV for Fe2+ and Fe3+, respectively, which indicates that Fe has two oxidation states.19) The Co peaks of FeCo-LDH-SO4 at 780.7 and 796.1 eV were identified as Co 2p3/2 and Co 2p1/2 of Co3+ species, respectively. The peaks at 784.1 and 798.2 eV were determined to be the Co2+ species [Fig. 5(c)]. The Co 2p spectrum of the corresponding LDH grown on NF also confirmed the presence of Co in the Co3+ and Co2+ oxidation states. The O1s spectrum consists of three peaks occurring at 529.1, 530.8, and 532.7 eV for oxide (M-O), hydroxide (M-OH), and adsorbed water groups,20) respectively [Fig. 5(d)]. Microstructural and spectral changes mean that the structural characteristics of compounds synthesized from different metal salts are significantly different, thus exhibiting different electrocatalytic properties.
Using a conventional three-electrode system in 1 KOH electrolyte, a series of electrocatalysts were tested for their electrocatalytic activity towards OER. FeCo-LDH-SO4 demonstrated excellent OER catalytic performance, requiring overpotentials of 247 mV to produce a current density of 10 mA cm-2. This overpotential is significantly lower than those of FeCo-LDH-Cl (252 mV), FeCo-LDH-NO3 (282 mV), and NF (370 mV) [Fig. 6(a)]. Moreover, it obtained overpotentials of 294 and 330 mV at current densities of 50 and 100 mA cm-2, much lower than those of FeCo-LDH-Cl, FeCo-LDH-NO3, and NF, as shown in Fig. 6(b). This demonstrates the controllability of different anionic iron salts on the electrocatalyst function. In addition, FeCo-LDH-SO4 has a Tafel slope of 60.6 mV dec-1, which is much lower compared to FeCo-LDH-Cl (66.1 mV dec-1) and FeCo-LDH-NO3 (69.1 mV dec-1), suggesting that the reaction kinetics are faster [Fig. 6(c)].21) FeCo-LDH-SO4 is an all-around high-quality OER catalyst with minimal overpotential and Tafel slope. Furthermore, electrochemical impedance spectroscopy (EIS) demonstrated employed to evaluate the charge transfer resistance (Rct) of various samples. According to the equivalent circuit diagram, the radius of the arc in the Nyquist plot represents the charge transfer resistance Rct. Generally, a smaller semicircle radius means lower charge transfer resistance and therefore a higher electrochemical reaction rate. The Rct of FeCo-LDH-SO4 (0.32 Ω) was significantly smaller compared with FeCo-LDH-Cl (0.39 Ω) and FeCo-LDH-NO3 (0.51 Ω), indicating that FeCo-LDH-SO4 has faster reaction kinetics and high conductivity [Fig. 6(d)]. The EIS results clearly show that the hybrid structure of nanowires and nanosheets makes electron transfer easier due to the shorter electron transfer distance.
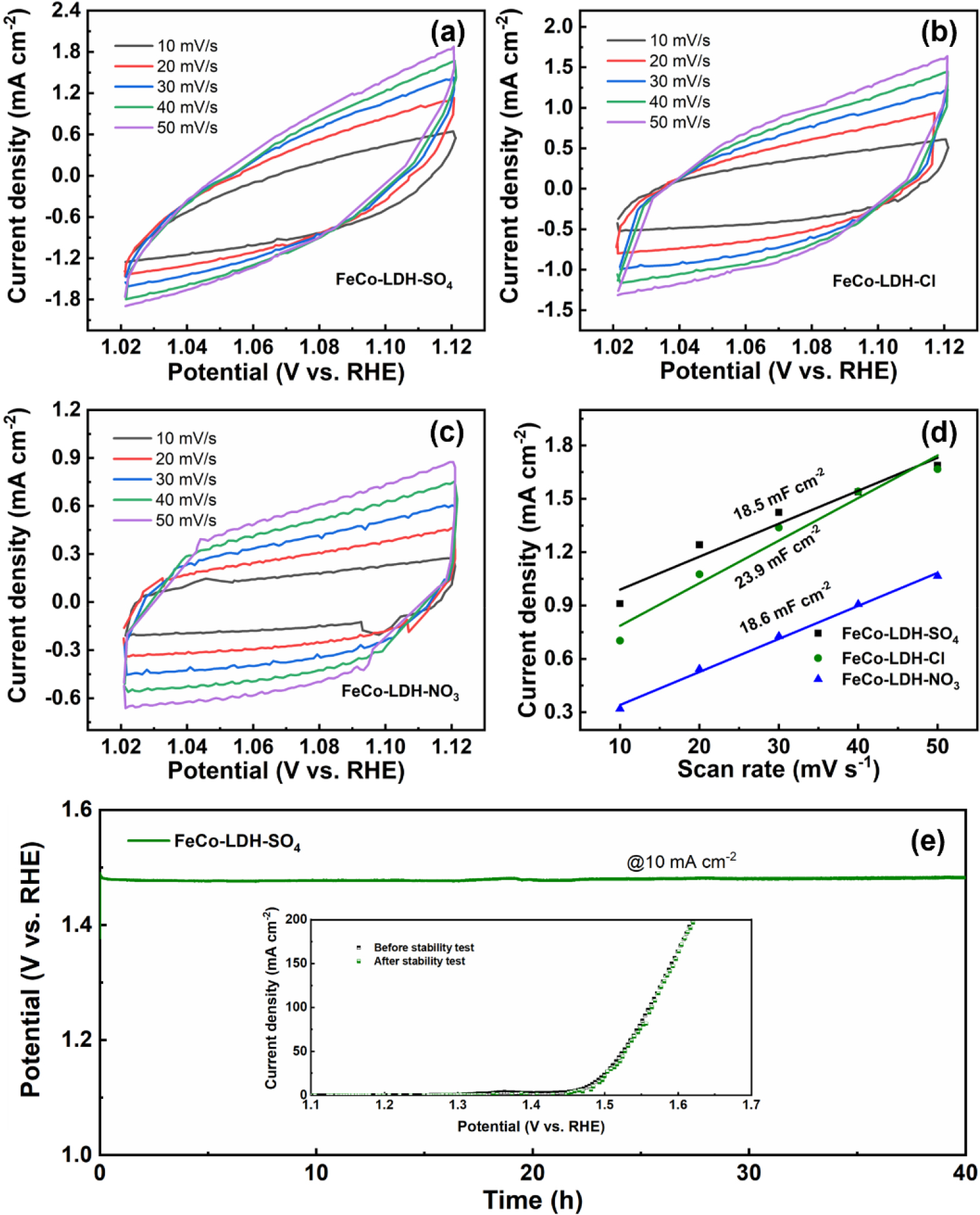
In addition, the electrochemical surface area (ECSA) of FeCo-LDH-SO4, FeCo-LDH-Cl, and FeCo-LDH-NO3 were investigated from the electrochemical double-layer capacitance (Cdl), and the Cdl of the electrode was determined by converting the cyclic voltammograms (CVs) in the non-faradaic range [Fig. 7(a-d)]. FeCo-LDH-Cl showed a higher Cdl value (23.9 mF cm-2) than FeCo-LDH-SO4 (18.5 mF cm-2) and FeCo-LDH-NO3 (18.6 mF cm-2), which may be due to its smaller nanoparticle size.
It is worth noting that ECSA and catalyst performance are not always positively correlated, and the high performance of FeCo-LDH-SO4 may be related to its high intrinsic activity rather than ECSA. To sum up, suitable ECSA and higher intrinsic activity can lead to better catalytic performance.22) Additionally, long-term stability is another crucial factor for assessing catalytic electrodes. As shown in Fig. 7(e), FeCo-LDH-SO4 exhibits excellent stability for 40 h with stable potential at 10 mA cm-2. The LSV curves of FeCo-LDH-SO4 before and after the CP test are shown in the inset in Fig. 7(e). No significant attenuation was observed, indicating excellent stability.
4. Conclusion
In summary, FeCo-LDH-SO4 nanowire and nanosheet hybrid structures were synthesized by a one-step ion exchange method using ZIF-67 as a template. The electrocatalysts formed by different anion iron salts have different electronic structures and different morphologies. FeCo-LDH-SO4 demonstrated the lowest overpotential (246 mV), low Tafel slope (60.6 mV dec-1), and excellent stability for over 40 h at a current density of 10 mA cm-2, indicating remarkable OER performance. The outstanding catalytic performance can be attributed to its unique hybrid structure, which not only accelerates the transfer of electrons but also prevents the collapse and aggregation of the nanostructure, thereby having higher electrochemical activity and stability. This work provides a new approach to synthesizing and tuning efficient, affordable, and stable OER LDH-based catalysts.