1. Introduction
2. Result and Discussion
2.1. Materials
2.2. Synthesis and characterization of zirconyl oxychloride (ZOC)
2.3. Preparation of zirconyl nitrate (Zr(NO3)4 feed solution
2.4. Extraction of Zr and Hf
3. Result and Discussion
3.1. Analysis and characterization of zirconium oxychloride (ZOC)
3.2. Extraction and separation of Zr and Hf
4. Conclusion
1. Introduction
In the domains of nuclear energy, metallurgy, the military, petrochemical, and novel materials science, zirconium is extensively used.1) The primary natural sources of zirconium used to make industrial products are zircon (ZrSiO4) and baddeleyite (ZrO2).2) Because zirconium has a very low thermal neutron absorption capacity, it is used in nuclear energy applications such nuclear fuel cladding. Because of its higher thermal neutron absorption cross-section more than 600 times greater than zirconium, hafnium is used as a control rod in reactors. Applying zircon sand (ZrSiO4) to nuclear materials can have a significant impact since it substitutes 1 % of the hafnium present in the crystal lattice for Hf.3) However, because Zr and Hf have comparable chemical characteristics, the separation of the two elements is difficult.4)
Zirconium and hafnium are extremely difficult to separate primarily because to their comparable chemical and physical properties (e.g., atomic radius, ionic radius and electronegativity.5,6,7,8) There are several known techniques for separating Zr from metals in solution media. Such methods include ion exchange/absorption,9,10) solvent extraction,11,12) reverse-phase liquid chromatography,13) and biosorption.14)
Several researchers have conducted effective research on the separation of Zr and Hf. Kabangu et al.15) succeeded in separating Zr and Hf using 2-octanol extractants (C8H18O). Zirconium and hafnium have a separation factor of 9.2 under these circumstances, and the highest extraction percentage is 96.58 %. Kabangu et al.16) carried out another study on the separation of Zr and Hf by extracting zirconium using 1-octanol. The organic phase is applied to recover zirconium. The best separation outcomes were achieved with 1.5 M KF and 10 % HCl concentrations. With a 2:1 organic to aqueous phase ratio, 15 min is the ideal reaction time. Hafnium (Hf) was extracted in HNO3 by He et al.17) using Cyanex 572 as an extractant. With an optimum extraction rate of 76.8 %, Hf can be extracted selectively, and the Hf and Zr separation factor can be as high as 15.7.
Wang et al.18) performed extraction of Zr and Hf with sulfuric acid media and used amine-based extractants. By using amines, zirconium is preferentially extracted via hafnium in the 0.1~3 M of H2SO4. In 0.5 M H2SO4 solution, alamine 308 yields the maximum separation factor of 12.4. Wang et al.19) used LIX®63, PC-88A, and a combination of PC-88A and LIX®63 in a solvent extraction investigation with nitric acid concentrations ranging from 0.5 to 4 M to separate the two metals. PC-88A produced greater extraction efficiency for both metals compared to LIX®63 (5,8-diethyl-7-hydroxydodecan-6-oxime), which produced 18.6 factor of separation for Zr and Hf. Wang et al.20) conducted separation of Zr(IV) and Hf(IV) from sulfuric acid solution by selective extraction has been carried out. Di-2-ethylhexylphosphoric acid (D2EHPA) was used to extract Hf(IV) from a sulfuric acid solution. Zr(IV) produced a separation factor of 10 when it was selectively extracted using D2EHPA in a strong sulfuric acid solution medium.
Zirconium and hafnium were extracted using a solvent in this investigation. In the solvent extraction procedure, zirconium and hafnium can be separated by transforming the metal into complex compounds that dissolve in the organic phase. The organic phase consists of ligand groups capable of selectively reacting with metallic elements in the aqueous phase. This separation occurs as a result of differences in the reactivity and diffusivity of each metal element to the organic phase. Organic extractants can selectively extract ions in acidic solutions.21) Using nitric acid, ZOC from the synthesis of Kalimantan zircon sand is dissolved to complete this technique. Then Zr and Hf were complexed using TBP-Dodecane extractants. Combination of TBP-Dodecane was used for this work because n-Dodecane gives a higher dipole moment for TBP extractant thereby increasing the roughness of the water interface as a result of reduced interface tension in the organic phase. Due to the interface, more water molecules are transferred to the organic phase so that the separation of Zr and Hf becomes more effective.22) Some of the extraction parameters studied in this research include variations in the organic and aqueous phase (O/A) 1:1; 1:2; 1:3; 1:4; and 1:5, contact time 15, 30, 45, 60, and 75 min, and nitric acid concentration 1, 3, 5, and 7 M.
2. Result and Discussion
2.1. Materials
The materials used in this study were beneficiated Kalimantan zircon sand concentrate, NaOH (Flakes 98 %, PT. Asahimas Chemical, Indonesia), HCl (37 %, Sigma-Aldrich, USA), acetone (70 %, CV. Alfa Kimia, Indonesia), HNO3 (65 %, Merck, Germany), tributyl phospate (99 %, Sigma-Aldrich, USA), n-Dodecane (99 %, Merck, Germany), tridodecylamine (97 %, Merck, Germany), sodium nitrate (Merck, Germany), hafnium standard (1,000 mg/L, Merck, Germany), and zirconium standard (1,000 mg/L, Merck, Germany).
2.2. Synthesis and characterization of zirconyl oxychloride (ZOC)
1,250 g of zircon concentrate was melted with 1,375 g of technical NaOH at 800 °C for 90 min. 1,350 g of melted zircon concentrate leached with 40.5 L of water at 60 °C with a stirring speed of 120 rpm for 60 min. 975 g of solids were leached using 19.5 L of 4 M HCl solvent at 90 °C with a stirring speed of 150 rpm for 90 min. The material obtained was analyzed for its composition using XRF Panalytical Type Epsilon 4, and characterized between samples of Merck ZOC and ZOC synthesized using XRD Panalytical Type Aeris and wavenumber characterization with FTIR Bruker Type Alpha II.
2.3. Preparation of zirconyl nitrate (Zr(NO3)4 feed solution
35.7 g of ZOC is then dissolved into 350 mL HNO3 7 M until solids dissolve. 148.75 g of NaNO3 was added little by little while stirring. The solution is diluted in a measuring flask of 500 mL.
2.4. Extraction of Zr and Hf
Extraction is carried out using a mechanical shaker. The feed solution used in this experiment was zirconium nitrate. Extraction is carried out by shaking at a variation in the O/A ratio of 1:1; 1:2; 1:3; 1:4; 1:5; 2:1; 3:1; 4:1; 5:1 with variations in shaking time (15, 30, 45, 60, and 75 min). 2 separate phases were obtained, namely the organic phase and the water phase. To find the concentration of Zr and Hf in the aqueous phase, this research using rigaku type XRF. Eq. (1) provides the ratio of the organic to the water phase concentration, which is used to calculate the value of the distribution coefficient (D). CA is the concentration of the aqueous phase and CO is the concentration in the organic phase. The values of extraction efficiency and separation factor are calculated based on Eqs. (2), (3) and (4). Vaq is the aqueous phase volume while Vorg is the organic phase volume, DZr and DHf are the distribution coefficients for Zr and Hf.
3. Result and Discussion
3.1. Analysis and characterization of zirconium oxychloride (ZOC)
To determine the quality of synthesis ZOC from Kalimantan zircon sand concentrate, a comparison of the results of the analysis of the elemental composition of Merck ZOC and synthesized ZOC with XRF was carried out, characterization using FT-IR to compare the wavenumber characters of Merck ZOC and synthesized ZOC, and XRD characterization to compare the diffractogram characters of Merck ZOC and synthesized ZOC.
3.1.1. Elemental composition analysis of ZOC
Analysis of the elemental composition of synthesized ZOC and Merck ZOC has been carried out to compare the results of the elements contained between the two materials. The dominant elements contained in ZOC are Zr, Cl, and Hf. Hf elements have a strong attachment to Zr, especially in nature. In Table 1, the results of Zr elements for Merck ZOC and ZOC synthesized are 59.915 % and 59.969 %, Cl elements for Merck ZOC and ZOC synthesized are 31.519 % and 33.436 %, while the Hf element in Merck ZOC and synthesized ZOC are 1.708 % and 1.581 %.
Table 1.
Element composition of Merck ZOC and synthesized ZOC.
3.1.2. FTIR analysis of ZOC
Analysis of Merck ZOC functional groups and feed ZOC for Zr-Hf separation extraction was carried out using the FTIR instrument, from the FTIR image obtained characteristic peaks appeared at wavenumbers 740~913 cm-1, 1,621 cm-1, 2,000 cm-1, and 3,200 cm-1. The peaks appearing at 740~913 cm-1 are characteristic wavenumbers for Zr-O-Zr,23) the peak at wavenumber 1,621 cm-1 is the characteristic peak for H2O absorption and the peak at wavenumber 3,200 cm-1 is the characteristic peak for O-H stretching.
3.1.3. XRD characterization of ZOC
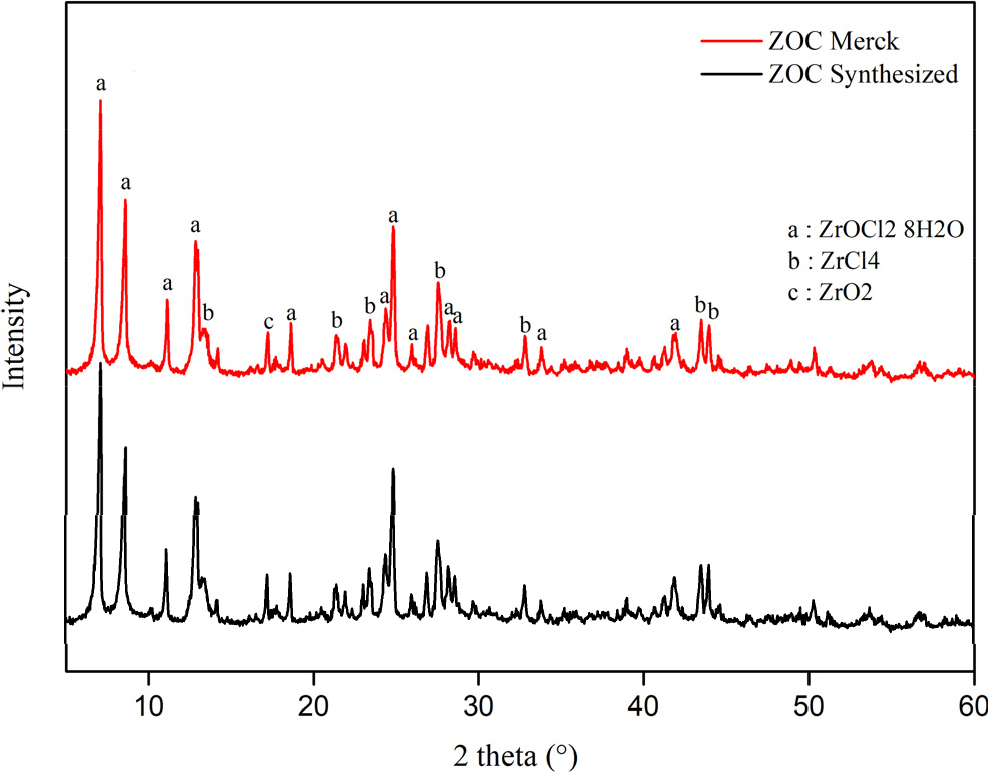
The purity of ZOC synthesized from Borneo zircon sand concentrate can be characterized using XRD tools. The XRD instrument uses a Cu anode at a scanning angle of 5~85°. Fig. 2 shows the comparison of diffraction patterns between ZOC Merck and ZOC synthesis. Based on Fig. 2, there is no significant difference between the diffraction pattern of Merck ZOC and synthesized ZOC. From the diffractogram obtained the characteristic peaks for the crystal phase ZrOCl2 ‧ 8H2O at 2θ 7.1434°, 8.6256°, 11.2479°, 12.7871°, 18.7043°, 24.4050°, 26.0126°, 28.2245°, 28.5665°, 33.7883°, and 41.9174°. The crystalline phase of ZrCl4 is shown at 2θ 13.3115°, 21.4521°, 23.4017°, 27.5974°, 32.7166°, 43.4794°, and 43.9697° and ZrO2 at 2θ 17.1652°. Fig. 2 shows no significant diffraction pattern difference between Merck ZOC and synthesized ZOC.
3.2. Extraction and separation of Zr and Hf
From the results of elemental composition analysis using XRF, characterization using FTIR and XRD, ZOC material has been successfully synthesized from the concentrate of Kalimantan zircon sand raw materials. This synthesized ZOC material will be used for Zr-Hf extraction feed and separation. To determine the optimal conditions for separation efficiency, the influence of experiments is examined, including the influence of the ratio of water phase and organic phase, the influence of acidity, the influence of speed and time of stirring contact.
3.2.1. Effect of ratio organic and aqueous phase (O/A)
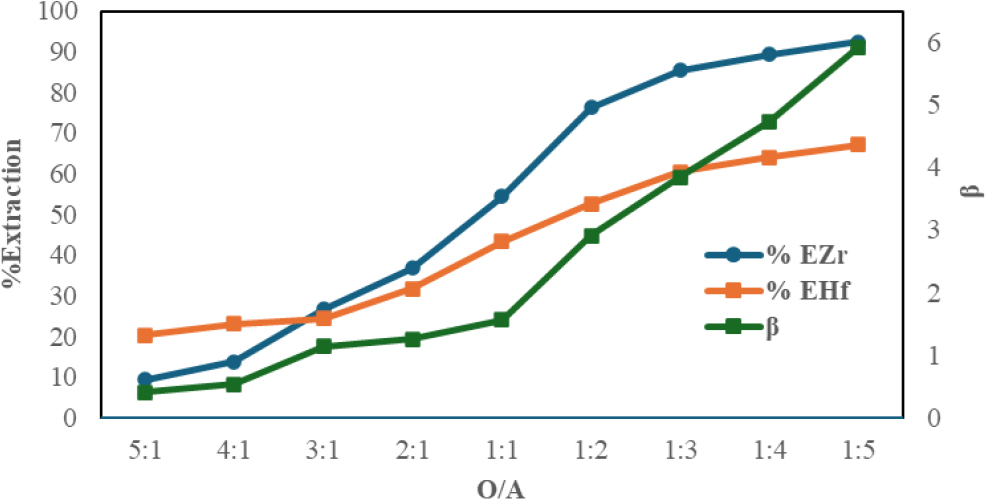
The effect of the ratio of organic and organic phase (O/A) was carried out in this study. The ratio of the aqueous phase and the organic phase varies at a ratio of 1:1; 1:2; 1:3; 1:4; 1:5; 2:1; 3:1; 4:1; and 5:1. Extraction was carried out at a stirring setting of 200 rpm for 75 min with a concentration of nitric acid used 1 M. TBP-Dodecane mixture as an organic phase was mixed with zirconium nitrate feed solution. In research conducted by Kabangu et al.15) stated that the use of TBP extractants can be combined with MIBK to prevent the formation of the third phase. In the current study, the use of TBP-Dodecane extractants does not form a third phase which can inhibit the separation process in determining the organic phase and water phase for the separation of Zr and Hf. From the results of the study, a significant %Zr extraction value was obtained which was 97.32 % when compared to the research conducted by Kabangu et al.16) when compared with research conducted by Kabangu et al.16) using 2-octanol extractants with hydrochloric acid media with a value of 96.58 % at an O/A ratio of 1:1. The use of organic phase composition is much greater when compared to the 1:5 O/A ratio for TBP-Dodecane extractants. In this study, a long carbon chain n-Dodecane was used which had a significant impact on the process of separating Zr and Hf. Previous research conducted by Wang et al.19) said that Among these three tertiary amines, the extraction order for both Zr and Hf is: Alamine 300 > Alamine 308 > TEHA. Based on this extraction order, it could be said that highly branched chains generally result in inefficient extraction due to steric effect. The extraction results to see the effect of the ratio of organic and aqueous phase are shown in Fig. 3 which indicates that the selection of the optimum ratio (O/A) is 1:5.
This shown that when the ratio of water phase volume to organic phase volume grows, it also increases the proportion of Zr and Hf extraction. While it decreases from 1:1 to 5:1 (O/A) ratio, the separation factor for Zr and Hf extraction rises from 1:1 to 1:5. Additionally, Janubia reported that using TBP extractants, increasing the nitrate volume ratio would enhance the proportion of Zr and Hf extraction.24) TBP-Dodecane is able to suppress the interfacial tension between the organic phase and the water phase. The greater the TBP-Dodecane ratio of an extraction medium, the water/polar molecules unable transport to the organic phase.21) The results showed the lowest D Zr value at O/A conditions 5:1. Table 2 shows data generated from experiments that have been conducted.
Table 2.
Effect of phase ratio (O/A) on Zr and Hf extraction. Experimental conditions: TBP-Dodecane (95 %:5 %), [HNO3] 1 M, Stirring Speed 200 rpm.
3.2.2. Effect of contact time
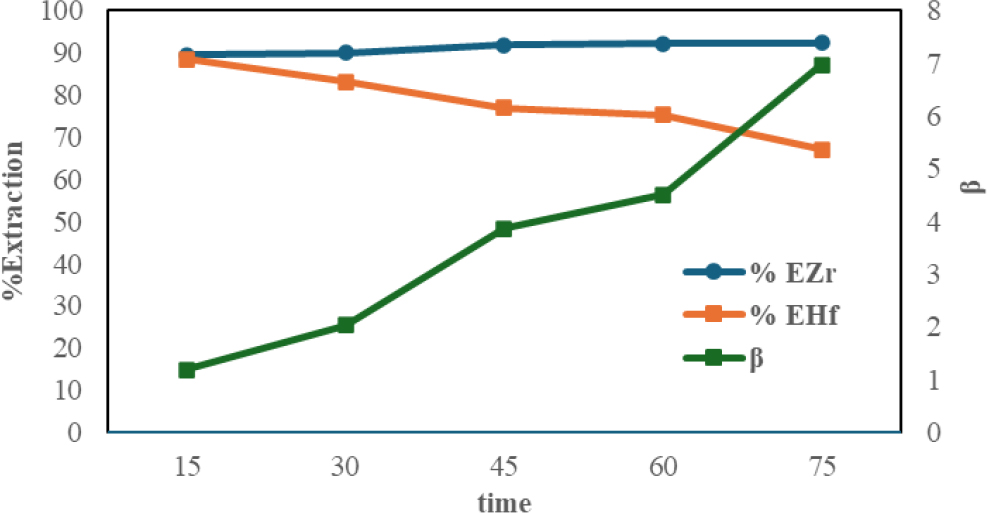
One of the factors needed to ensure that a separation process between Zr and Hf is succesfully carried out is contact time. The extraction process shown in Table 3 is carried out using the optimum phase ratio of O/A (1:5) for time variations between 15~75 min.
Table 3.
Effects of contact time on Zr and Hf extraction. Experimental conditions: O/A 1:5, [HNO3] 1 M, stirring speed 200 rpm.
| Extraction time (minute) | D Zr | D Hf |
| 15 | 1.8072 | 1.5156 |
| 30 | 1.8614 | 0.9186 |
| 45 | 2.4234 | 0.6264 |
| 60 | 2.4653 | 0.5464 |
| 75 | 2.5842 | 0.3705 |
The extraction percentage as a function of time is shown in Fig. 4. The maximum values for the best extraction percentage and separation factor were shown at 75 min with %EZr in the maximum organic phase at 92.34964 % and %EHf in the minimum phase at 67.10311 %.
3.2.3. Effect of HNO3 concentrations
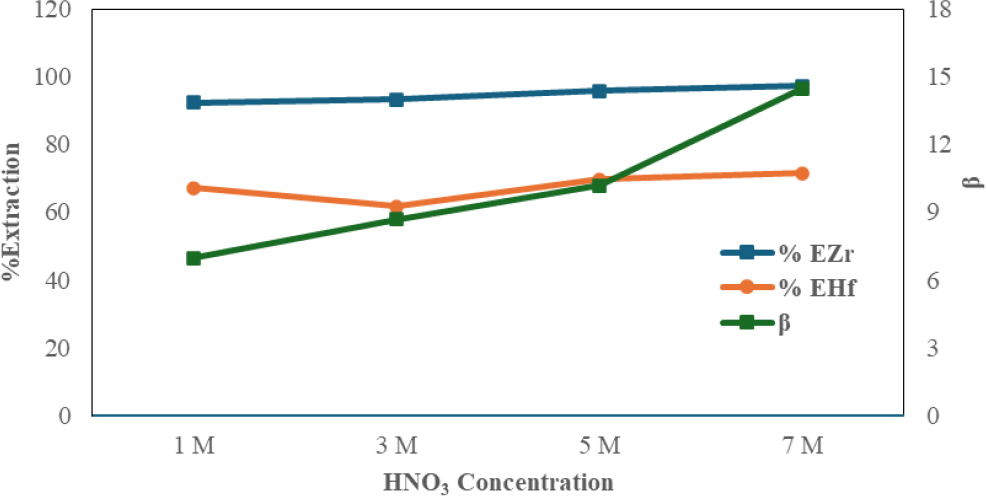
The impact of varying nitric acid concentration on Zr and Hf extraction is investigated. Nitric acid is used to dissolve zirconium oxychloride at different concentrations ranging from 1 to 7 M. The use of nitric acid as water phase of extraction is considered better than using hydrochloric acid based on research conducted by Biyantoro et al.21) NaNO3 is added to the solution after it has dissolved. Wang et al.20) in their research separated Zr and Hf in nitric acid medium using NaNO3 to increase the value of separation factor. During the extraction process, the feed solution comes into contact with the organic phase of TBP-Dodecane, allowing Zr and Hf to be separated. The test was conducted with an O/A ratio of 1:5 with a contact time of 75 min. The results shown in Fig. 5 indicate that as nitric acid concentration increases, separation increases (this is shown in the β value). It can be explained that increasing acid concentrations result in the promotion of depolymerization due to the presence of OH- ions that are simultaneously captured by Zr or Hf.17) Therefore, the better the Zr and Hf extraction with TBP-Dodecane, the greater the nitric acid concentrations. Studies conducted by Wang et al.18) showed that the percentage of Zr extraction increases according to the increase in sulfuric acid. this is due to the hydrolysis effect of Zr on the formation of metal complexes. The formation of Zr hydrolysis occurs at a pH of about 0.5. This is related to the research that has been done, Zr and Hf separation is positively impacted by increasing the HNO3 concentration (seen from the value of β).
From the calculation results, a concentration of 7 M is the optimum concentration because the highest separation value is 14.48874. From the data in Table 4, it is known that the value of Zr extraction percentage is increasing while Hf is decreasing. This is what influences the increase in the value of the separation factor Zr and Hf. Hf has a weaker tendency than Zr to form anionic complexes, this is confirmed according to the research of Poriel et al.25) They clarify that Zr is harder than Hf.26) This suggests that Zr will be more attached to the extraction process utilizing anion liquid extraction, a hard base NO3-, in the TBP-Dodecane organic phase. The increase in the value of the separation factor is influenced by the solvation reaction responsible for the extraction process between the two metals.
Table 4.
Effects of nitric acid concentration variations on Zr and Hf extraction. Experimental conditions: O/A 1:5, contact time 75 min, stirring speed 200 rpm.
| HNO3 | D Zr | D Hf |
| 1 M | 2.5842 | 0.3705 |
| 3 M | 2.7979 | 0.3224 |
| 5 M | 4.6681 | 0.4588 |
| 7 M | 4.0312 | 0.2782 |
Zirconium and hafnium are primarily found as oxycations MO2+ in moderately acidic aqueous solutions, while MO(NO3)2 is the predominant species in nitric acid media. This demonstrates that, according to the solvation approach, organophophorus extractants are neutral metal ions. The suggested reaction from the above is expressed in the following equation:
where, MO2+ represents cationic species Zr and Hf in HNO3 and L solutions as TBP-Dodecane extractants.27) The speciation of the metal complex formation is influenced by the nitrate concentration, and the increase in nitrate concentration results in the creation of the extracted metal-nitrate complex in this case shown in Fig. 5. At the concentration of HNO3 7 M, the optimum Zr and Hf separation value is 14.48874.
4. Conclusion
Using TBP-Dodecane in HNO3 medium as an extractant, zirconium and hafnium have been extracted and separated. Extraction is carried out using a mechanical shaker according to the parameter conditions. The results showed that zirconium was separated from hafnium at optimum conditions at organic to aqueous ratio of 1:5, a contact time of 75 min, and HNO3 concentration of 7 M. The resulting separation factor was 14.4887.