1. Introduction
2. Experimental Procedure
2.1. Reagents and materials
2.2. Preparation of miniemulsion
2.3. Preparation of microemulsion
2.4. Polymerization
2.5. Measuring instruments and methods
3. Results and Discussion
3.1. Analysis by SEM
3.2. Spectral transmittance
3.3. Refractive index and water content
3.4. Oxygen permeability
3.5. Contact angle and AFM analysis
3.6. Polymerization stability
4. Conclusion
1. Introduction
Currently, many people are using glasses and contact lens for vision correction. In particular, contact lens are in increasing demand due to their vision correction, convenient fit, and excellent beauty products.1) The main material of hydrogel lens is 2-Hydroxyethyl methacrylate (HEMA). HEMA has excellent water content, wettability, and biocompatibility, but hypoxia causes eye diseases such as corneal edema and keratitis.2) Accordingly, a silicone hydrogel contact lens with improved oxygen permeability by adding a silicone monomer was developed to solve hypoxia.3) Silicone monomers have high oxygen permeability, but their wettability decreases because they are hydrophobic.1) Furthermore, the silicone monomer is not smoothly stirred when polymerized with a hydrophilic monomer such as HEMA. Therefore, phase separation occurs, causing opacity of the lens.2) Accordingly, research is continuously being conducted to compensate for shortcomings and maintain advantages of silicone hydrogel lens as much as possible.1,4) Amino modified silicone oil (AMSO) is a silicone oil containing NH2 (amino group) in addition to CH3 that general silicone oil has. It has a high oxygen permeability and has hydrophobic properties. In addition, it has a hydrophilic group NH2 and has higher water content and wettability than silicone oil, so it is widely used as a leather and fiber softener. Emulsions are mainly a state in which two liquids which do not mix with each other, such as water and oil, are spread in a liquid form in a particle state in another liquid form.5) Emulsions are divided into macro emulsions (1,000 to 10,000 nm), miniemulsion (100 to 1,000 nm), and microemulsion (10 to 100 nm) depending on the size of the particles.6) Miniemulsion have flow characteristics and thus have excellent storage stability. It is used in various fields such as drug delivery systems (DDS), cosmetics, pharmaceuticals, and chemicals.6,7) Microemulsions have a thermodynamically very stable state and thus do not cause aggregation between them.8) In addition, the characteristics vary depending on the thermodynamic variables, and particles formed in equilibrium appear in the form of various geometric structures such as worm shape, sponge shape, hexagonal shape, spherical michelle.9) Emulsion has been studied a lot in various fields such as cosmetics, pharmaceuticals, synthesis of substances, nanoparticle manufacturing, and phase separation.6,10,11,12) However, there are remarkably few studies used in hydrogel lens. Therefore, this study compared and analyzed the physical properties of contact lens by applying AMSO to the basic copolymerization method and mini and microemulsion technique. Through this, it is intended to maximize the function of silicone hydrogel contact lens and increase compatibility.
2. Experimental Procedure
2.1. Reagents and materials
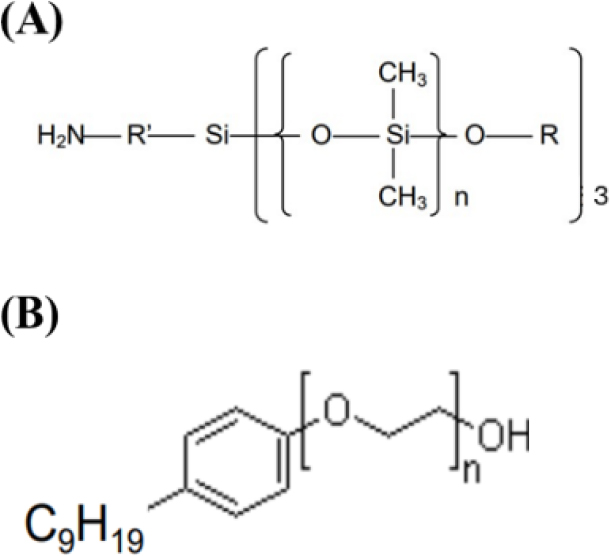
To manufacture silicone hydrogel lens, HEMA, the main material of hydrogel lens, and 1-Vinyl-2-pyrrolidinone (NVP), N,N-Dimethylacetamide (DMA), and AMSO (CS CHEM), methacrylic acid (MA, JUNSEI), cross-linker ethylene glycol dimethacrylate (EGDMA), and initiator azobisobutyronitrile (AIBN) were used as basic combinations. To manufacture miniemulsion, deionized water (DIW), co-stabilizer n-Hexadecane (HD), surfactant NP-2 (green chemical), initiator ammonium persulfate (APS), and AMSO were used. To manufacture microemulsion, DIW, surfactant NP-2, AMSO, acetic acid (AA), sodium hydroxide (NaOH), and propylene glycol (PG) were used. All reagents except AMSO and MA and NP-2 were used after purchase from Sigma-Aldrich.
2.2. Preparation of miniemulsion
The experiment was modified with reference to Kwon et al.13) In this study, the sample used as a monomer in the above paper was used as AMSO, and potassium persulfate (KPS) used as an initiator was used as APS. First, AMSO and HD, a co-stabilizer, were stirred for 15 min using vortex. After that, the stirred sample was added to DIW in which the surfactant was dissolved, and stirred for 20 min. The sample was homogenized for 15 min in an ultrasonic machine containing ice. To the homogenized sample, APS, which is an initiator, was added and stirred for 20 min again. After stirring, the mixture was reacted at 70 °C for 3 h. The mixing ratio is shown in Table 1.
Table 1.
Compositions of miniemulsion (unit: %).
| Sample | DIW | HD | NP-2 | APS | AMSO1) | Total |
| Miniemulsion | 70.33 | 1.01 | 0.50 | 0.03 | 28.13 | 100.00 |
2.3. Preparation of microemulsion
The experiment was modified with reference to Patent Application WO2011025325.14) The preservative added in the above paper was not used in this study, and the experiment was conducted by arbitrarily determining the stirring time. First, surfactant and AMSO were added to DIW, followed by stirring for 20 min. After that, the stirring speed was lowered, AA was added, and the mixture was stirred again for 20 min. After adding 15 M NaOH to the sample while maintaining the stirring, the temperature was raised to 50 °C for 30 min. After raising the temperature, it was cooled to 40 °C or less. After cooling, PG was added. The mixing ratio is shown in Table 2.
Table 2.
Compositions of microemulsion (unit: %).
| Sample | DIW | NP-2 | AMSO1) | AA | NaOH | PG | Total |
| Microemulsion | 65.56 | 9.01 | 23.02 | 0.35 | 0.06 | 2.00 | 100.00 |
2.4. Polymerization
For polymerization of the lens, HEMA, NVP, DMA, AMSO, MA, EGDMA, and AIBN were used as basic combinations. AMSO, mini and microemulsion were used after adding MA and reacting at 800 rpm for 10 min at an elevated temperature of 50 °C, respectively. Samples used in the experiment were named REF, A, B, and C, respectively. The sample was stirred using ultrasonic waves 1 h 20 min and vortex 30 min, and heat polymerized at 100 °C for 2 h using a cast mold method. Table 3 shows the mixing ratio for the preparation, and the chemical structural formula of AMSO and NP-2 used in this study is shown in Fig. 1.
Table 3.
Percent compositions of the silicone hydrogel lens samples (unit: wt%).
2.5. Measuring instruments and methods
The manufactured contact lens were hydrated in sodium chloride (NaCl) physiological saline at a concentration of 0.9 % for 24 h and then optical and physical properties were evaluated through spectral transmittance, refractive index, water content, oxygen transmittance, contact angle, atomic force microscope (AFM) and scanning electron microscope (SEM) measurements. Spectroscopic transmittance was measured according to ISO 8599:1994. Refractive index and moisture content were measured according to ISO 18369-4:2006, and water content was analyzed using a weight measurement method. Oxygen transmittance was measured by maintaining a temperature of 35 ± 0.5 °C and a humidity of 98 ± 1 % according to ISO 18369-4:2006. Contact angle was measured by the sessile drop method using a contact angle meter (Phoenix-300 Touch, SEO, Korea), and AFM analyzed the wettability and roughness of the lens using a nuclear force microscope (XE-100, Park Systems, Korea). The SEM observed the shape and size of the nanoparticles using FE-SEM (JSM-7500F+EDS, JEOL, Oxford). In addition, polymerization stability was evaluated by measuring KMnO4 reduction test, pH test, and absorbance in order to evaluate stability. For all measurement results presented in this study, all samples were repeatedly measured five or more times to increase the accuracy of the experiment. The comparison and analysis of samples were expressed as the measured average value.
3. Results and Discussion
3.1. Analysis by SEM
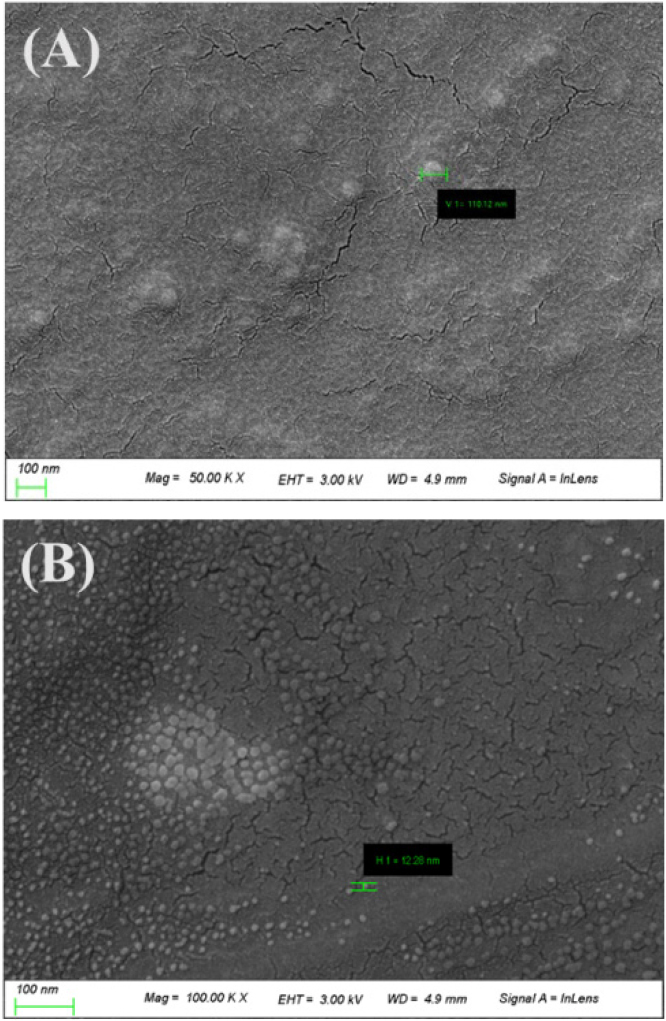
Measurements were made using SEM to confirm the surface and nanoparticles of the silicone hydrogel lens using the mini and microemulsion. As a result of SEM analysis, nanoparticles of 110.12 nm were observed in the contact lens using the miniemulsion technique, and nanoparticles of 12.28 nm were observed in the contact lens using the microemulsion technique. The SEM image of each sample is shown in Fig. 2.
3.2. Spectral transmittance
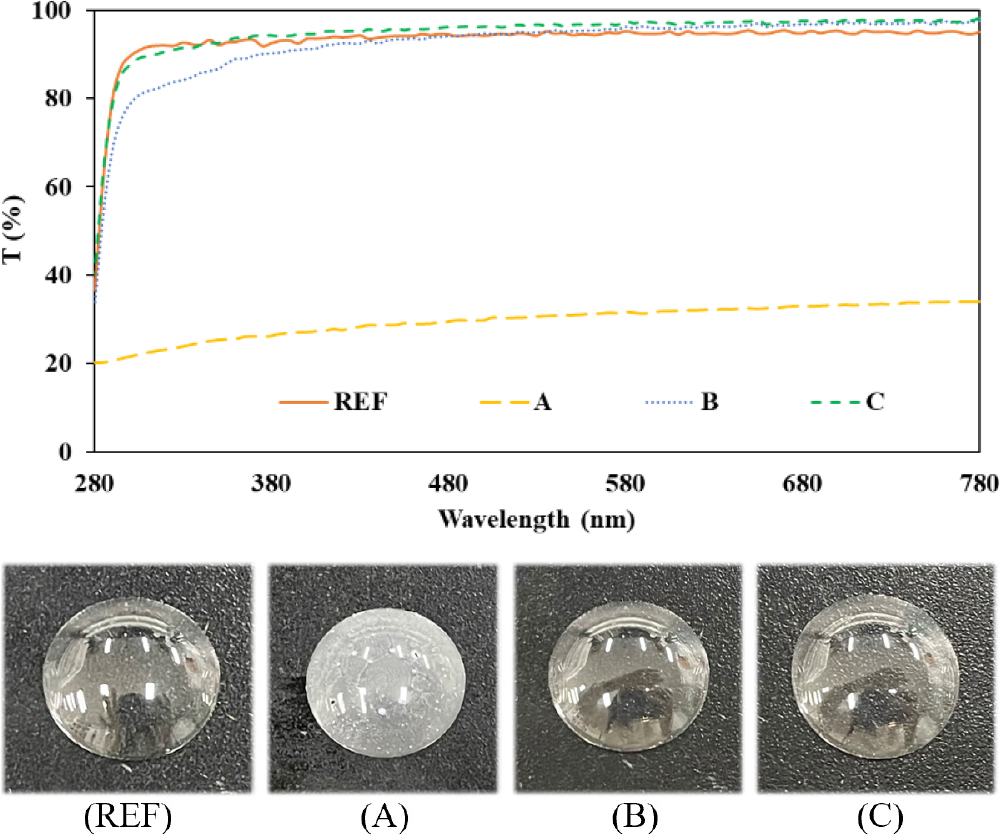
The spectral transmittance was measured by classifying it into UV-B (280~315 nm), UV-A (315~380 nm) and visible light region (380~780 nm). As a result of the measurement, sample A using the copolymerization method showed 31 % of the visible ray region, and samples B and C showed 90 % or more of the visible ray region. Accordingly, unlike sample A, samples B and C are judged to satisfy the basic characteristics as a contact lens by meeting more than 88 % of the basic contact lens requirement of ANSI Z80.20:2004. On the other hand, sample A does not satisfy 88 % or more, so it is judged that it has no compatibility and utilization as a contact lens. The graph of the spectral transmittance of each sample and the image of each sample are shown in Fig. 3.
3.3. Refractive index and water content
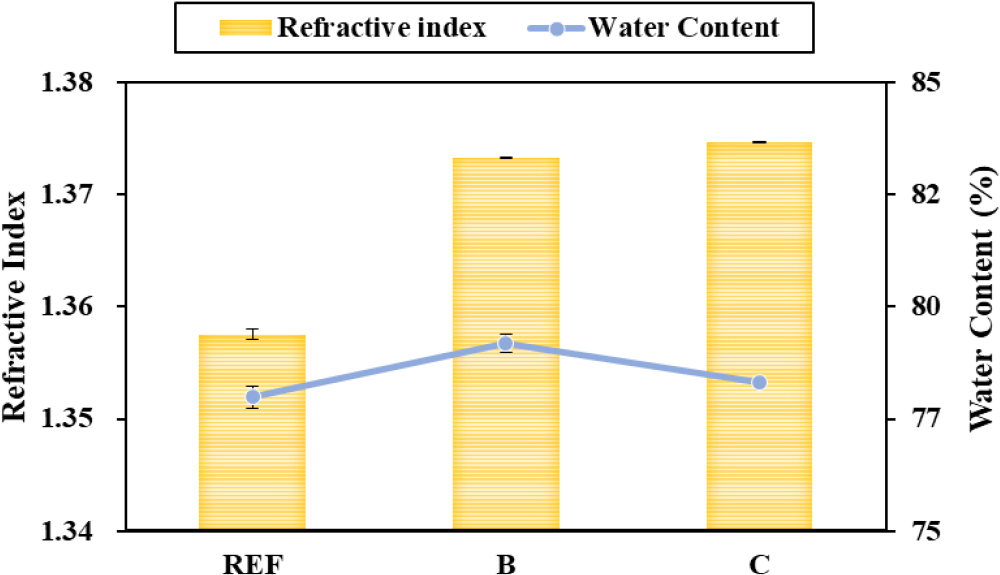
As a result of measuring the refractive index and water content of the manufactured silicone hydrogel lens, the refractive index of REF was 1.3575 and the water content was 77.48 %. In the case of contact lens using each emulsion manufacturing technique, the refractive index of B was 1.3733 and the water content was 78.70 %, the refractive index of C was 1.3747, and the water content was 77.82 %. Accordingly, all samples showed higher refractive index and water content than REF. the refractive index was higher in C, and the water content was higher in B, confirming that the refractive index and water content of the two samples were inversely proportional. The refractive index and water content of each sample are shown in Fig. 4.
3.4. Oxygen permeability
As a result of measuring the oxygen permeability of the prepared silicone hydrogel lens, REF was 32.21 × 10-11 (cm2/s) (mlO2/ml × mmHg). B was 37.04 × 10-11 (cm2/s) (mlO2/ml × mmHg), and C was 35.56 × 10-11 (cm2/s) (mlO2/ml × mmHg). Accordingly, all samples showed higher oxygen permeability than REF, and B showed higher oxygen permeability than C. It is determined that the oxygen permeability increases according to the increase in the water content.15)
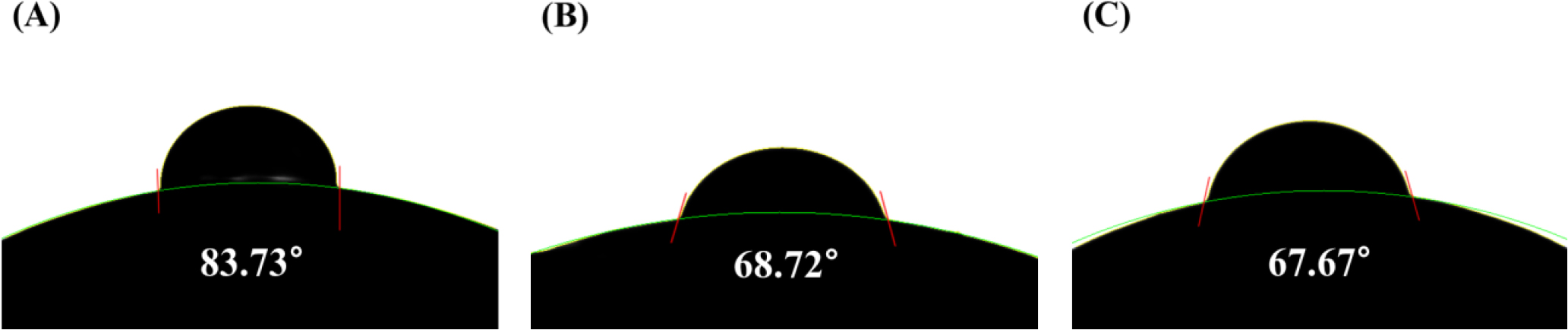
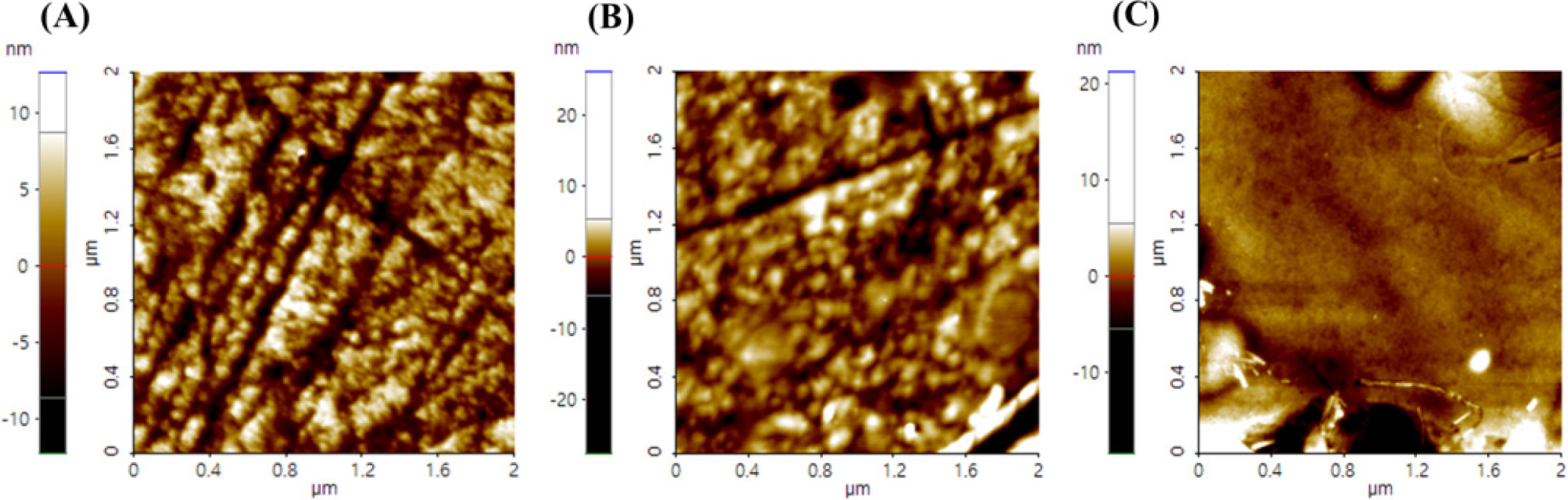
3.5. Contact angle and AFM analysis
As a result of measuring the contact angle and AFM of the hydrogel lens prepared for wettability and roughness evaluation, the contact angle of REF was 83.73°. B was found to be 68.72° and C was found to be 67.67°. Accordingly, all samples had a lower contact angle than REF, resulting in higher wettability, and it was found that C had a lower contact angle than B. It is judged that the wettability increased by reducing the surface’s interfacial tension by forming particles smaller than miniemulsion and forming many micelles.7) The contact angle image of each sample is shown in Fig. 5. In addition, the average surface roughness value (Ra) of the lens was confirmed to be REF 3.17 µm, B 1.97 µm, and C 1.62 µm, respectively. AFM was similar to the result of the contact angle. It is known that the surface roughness of a lens affects the contact angle.16) The AFM image of each sample is shown in Fig. 6.
3.6. Polymerization stability
In order to evaluate the polymerization stability of the copolymerization method, mini and microemulsion technique, KMnO4 reduction test, pH test, and absorbance were measured, respectively. The experiment was carried out with the eluate extracted by heating the lens at 70 °C for 24 h as an experimental group and the DIW as a control group. As a result of measuring the absorbance, light was absorbed the most at 235 nm. In particular, in the case of microemulsion, the lowest value was shown, confirming that the polymerization stability was the best. In addition, it was confirmed that the elution was not performed in all samples from the 5th day. The pH test showed that the difference between the control group and the experimental group in all samples was less than 1.5, showing excellent polymerization stability regardless of the manufacturing technique. In the KMnO4 reduction test, the extracted gum solution of the control group was 20.08, and the difference in the appropriate amount from the experimental group using the mini and microemulsion technique was measured to be less than 2 mL. However, in the experimental group using the copolymerization method, the difference in the appropriate amount exceeded 2 mL. Therefore, it is judged that the sample using the emulsion technique has better polymerization stability than the sample using the copolymerization method. The result values of the pH test and the KMnO4 reduction test are shown in Table 4.
4. Conclusion
In this study, AMSO was added to hydrogel contact lens using the copolymerization method, mini and microemulsion technique, respectively, and then the physical properties of the silicone hydrogel contact lens were compared and analyzed. As a result of measuring the physical properties of the contact lens according to the manufacturing technique, the lens using the copolymerization method did not satisfy the basic optical and polymerization stability characteristics. However, the lens using the mini and microemulsion technique satisfied optical properties and polymerization stability, and showed better refractive index, water content, wettability, and oxygen transmittance than REF without AMSO. Therefore, silicone hydrogel lens using mini and microemulsion technique are effective in improving stability and performance of hydrogel contact lens.