1. Introduction
2. Experimental Procedure
2.1. Hydrogel contact lens
2.2. Hydrogel contact lens containing gold nanoparticles
2.3. Surface modification of gold nanoparticles
2.4. Analysis
3. Results
3.1. Properties based on the shape of gold nanoparticles
3.2. Properties based on surface-modified gold nanoparticles
4. Discussion
5. Conclusion
1. Introduction
Nanoparticles are defined as particles with a range of 10 to 1,000 nm, and as they become known to have unique characteristics compared to existing micrometer-unit particles, research has been actively conducted to identify the characteristics of nanoparticles and use them in a variety of fields.1) Among them, the surface plasmon resonance of metal nanoparticles can be adjusted by varying not only the type of metal but also the particle size and shape, and their properties can change accordingly, so they are applied to various material fields.2) Among metal nanoparticles, gold (Au) nanoparticles, which are most commonly used, have different optical properties depending on the size and shape of the nanoparticles.3,4) Gold nanoparticles exist in many forms such as spherical nanoparticles, nanostars, nanocubes, nanotriangles, nanorods, nanowires, and nanoshells, and are used in a variety of ways in biomedical fields because they have characteristics that vary depending on their shape. Gold nanoparticles have numerous advantages such as improved tensile strength and antibacterial properties in the case of being applied to hydrogels.5) In addition, gold nanoparticles are attracting attention because they have excellent biocompatibility and can introduce various surface functional groups.6) Despite these advantages, gold nanoparticles have disadvantages as a material for contact lenses due to their own surface hydrophobic properties. According to Salih et al.,7) when gold nanoparticles were added to the contact lens alone without additives, a decrease in wettability of the contact lens was reported. Wettability is one of the important characteristics of contact lenses and it directly affects the fit, and wearing contact lenses with low wettability can make the wearer feel foreign matter.8) In addition, contact lenses with low wettability increase tear protein deposition, which causes eye diseases.9) The surface of the hydrophobic nanoparticles can be modified in various ways, and in particular, research on the surface modification of nanoparticles using a surfactant has been actively conducted.10) Hexadecyltrimethylammonium bromide (CTAB), which is a cationic surfactant, is used to modify the surface of nanoparticles, and can modify the hydrophobicity and hydrophilicity of the surface depending on the concentration.11) Surface modification with CTAB can form a single layer in which the hydrophobic alkyl chain is exposed through electrostatic interaction with Br-differentiated gold nanoparticles of CTAB at a certain concentration, and a hydrophilic surface can be formed by forming a phospholipid bilayer at a high concentration.12)
In this study, the properties were evaluated using two different gold nanoparticles, and the wettability and various properties of contact lenses were evaluated with CTAB on the surface of gold nanoparticles requiring surface modification. Therefore, it was intended to evaluate the use of gold nanoparticles as an ophthalmic material with the method of shape and surface modification.
2. Experimental Procedure
2.1. Hydrogel contact lens
In this study, for the manufacture of contact lenses, 2-hydroxyethyl methacrylate (HEMA) (purity: ≥99 %) and a hydrogel-based solution was prepared using 2,2'-Azobis (2-methylpropionitrile) (AIBN) (purity: 98 %) as a thermal initiator and ethylene glycol dimethacrylate (EGDMA) (purity: 98 %) as a crosslinking agent.
AIBN used products from JUNSEI (Japan), and HEMA and EGDMA used products from Aldrich (USA). All the mixed solutions used in this study were used after stirring for 30 min each with a vortex and an ultrasonic stirrer. Thermal polymerization was performed at 100 °C for 1 h and was prepared using a cast molding method. The lens of the basic hydrogel sample prepared without additives was named Ref, and all mixing ratios used in this experiment are shown in Table 1.
Table 1.
Percent compositions of hydrogel material samples (wt%).
2.2. Hydrogel contact lens containing gold nanoparticles
In order to prepare a hydrogel lens including gold nanoparticles, differently shaped (spherical, sea urchin-shaped) gold nanoparticles with a diameter of 50 nm were used. The gold nanoparticles were manufactured by Aldrich (USA). They were prepared in the form of concentrated pellets by centrifuging a dispersion dispersed in a phosphate buffer solution at a concentration of 0.1 mM at 14,000 rpm for 15 min. The prepared spherical gold nanoparticles were named AuNS, and the sea urchin-shaped gold nanoparticles were named AuNU. Each gold nanoparticle was added to the hydrogel-based solution in a ratio of 1 to 10 % to prepare a contact lens. Samples to which AuNS was added by ratio were named AuNS1, AuNS5, and AuNS10, and samples to which AuNU gold nanoparticles were added by ratio were named AuNU1, AuNU5, and AuNU10. In addition, the mixed solution showed different colors depending on the shape of the nanoparticles, and images of each mixed solution are shown in Fig. 1.
2.3. Surface modification of gold nanoparticles
Sea urchin-shaped gold nanoparticles prepared for the surface modification of nanoparticles and CTAB, a cationic surfactant, were used. CTAB was purchased from Aldrich (USA). To 10 mL of 0.5 M CTAB aqueous solution, 2 mL of sea urchin-shaped gold nanoparticles were added, and the mixture was stirred magnetically at room temperature for 15 h. The stirred solution was centrifuged at 8,000 rpm for 10 min. The precipitated sea urchin-shaped gold nanoparticles were redisperse in deionized water, and then repeated centrifugation was performed to remove unreacted surfactants. The prepared surface-modified sea urchin-shaped gold nanoparticles were named SMNU. Then, to prepare a contact lens including the surface-modified gold nanoparticles, SMNU was added at ratios of 1 %, 5 %, and 10 % to prepare contact lenses, which were named SMNU1, SMNU5, and SMNU10, respectively.
CTAB molecules can form surface ionic bonds with Br- ions attached to the Au surface, and the hydrophobic tail chain of CTAB faces outward as the head of the cationic CTAB surrounds the Br- layer by electrostatic interaction. In high concentration CTAB, it is adsorbed on the surface of gold nanoparticles in a double layer through a hydrophobic bond interaction according to the carbon chain.12) Therefore, in this study, the following principle of surface modification through the formation of the double layer was used, and the properties of the surface-modified contact lens were confirmed through the hydrophilic coating forming the outermost surface. The schematic diagram of surface modification of the nanoparticles is shown in Fig. 2.
2.4. Analysis
The prepared contact lens sample was hydrated in 0.9 % sodium chloride physiological saline for 24 h and used in the experiment, and optical and physical properties were evaluated. Optical transmittance was measured using Agilent (Cary 60 UV-vis, Santa, Clara, CA, USA), UV-B region (280~315 nm), UV-A region (315~380 nm), and visible light region (380~780 nm). The refractive index was measured by a weight measurement method using an Abbe refractometer (Atago, Japan), and the water content was measured by using an Ohaus electronic scale (PAG 214C, USA). Tensile strength was measured using an AGS-X Universal Testing Machine (Shimadzu, Kyoto, Japan), and the contact angle was measured with the sessile drop method using a DSA30 (Kruss GMBH, Germany) instrument. In addition, a scanning electron microscope (SEM) was used to confirm the shape of the nanoparticles, and surface roughness analysis was performed with an atomic force microscope (AFM). To evaluate the stability of the prepared contact lens, absorbance, which is an eluate test, was measured. This was done using the same machine as the optical transmittance instrument, and after hydrating in 10 mL of tertiary distilled water for 24 h, a sample solution was collected and measured. The average value of all experiments was calculated and used after measuring five times per sample to increase reliability.
3. Results
3.1. Properties based on the shape of gold nanoparticles
3.1.1. Optical transmittance
As a result of measuring the optical transmittance, Ref was found to be 96.63 % in the visible light region, 95.18 % in the UV-A region, and 81.67 % in the UV-B region. In the case of samples containing AuNS by ratio, the visible light region was 96.55 to 94.53 %, the UV-A region was 95.19 to 92.31 %, and the UV-B region was 81.26 to 79.27 %. For samples containing AuNU, optical transmittance in the visible light region ranged from 95.11 % to 96.59 %, the UV-A region was 94.88 to 93.01 %, and the UV-B region was 81.26 to 79.04 %. The optical transmittance of the visible light region of all the manufactured contact lenses was more than 88 % (ANSI Z80.20), showing high transparency optical properties.
3.1.2. Refractive index and water content
As a result of measuring the refractive index and water content, Ref was 1.4351 and 38.04 %, respectively. In the case of samples containing AuNS by ratio, the average was 1.4344 and 38.52 %, and in the case of samples containing AuNU by ratio, the average was 1.4344 and 38.37 %. There was no significant effect on the results of the refractive index and water content regardless of the shape of the gold nanoparticles.
3.1.3. Tensile strength
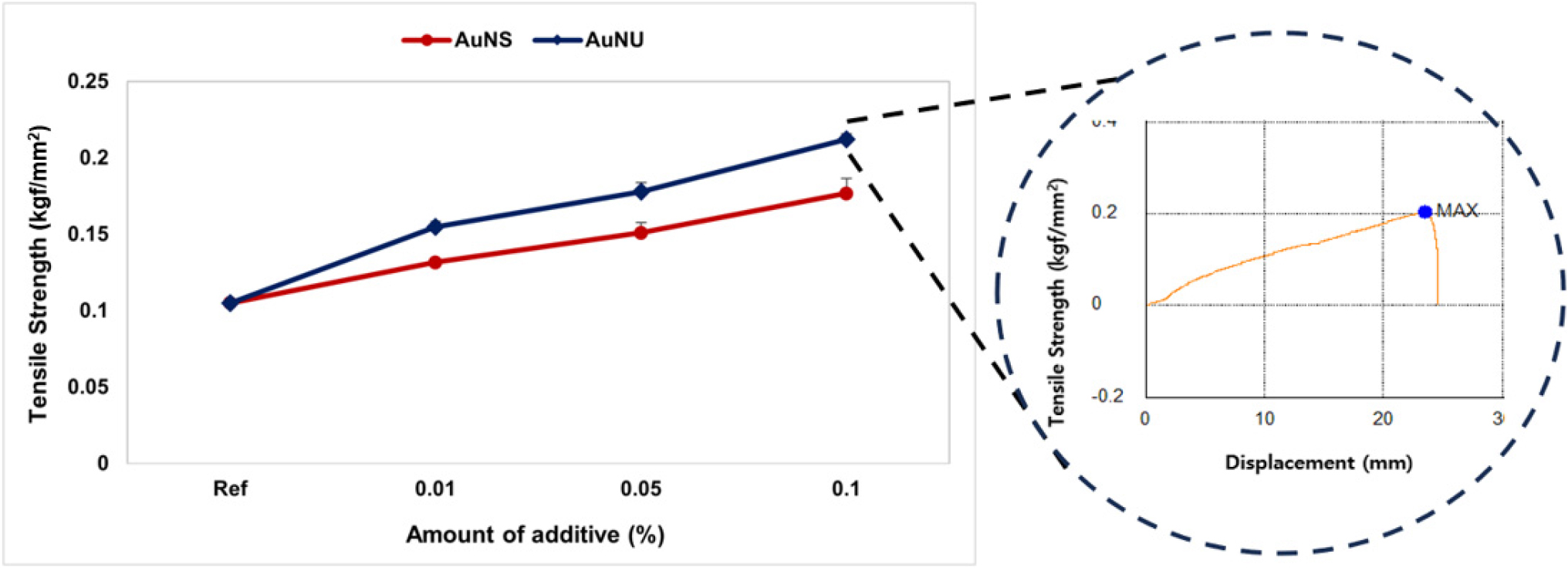
As a result of measuring the tensile strength, Ref was found to be 0.1049 kgf/mm2. The tensile strength of the sample to which AuNS was added was found to be 0.1318~0.1768 kgf/mm2 as the addition ratio increased. The tensile strength of the sample to which AuNU was added was 0.1547~0.2123 kgf/mm2 as the addition ratio increased. Each sample with gold nanoparticles added was 25.64 to 68.54 % in AuNS and 47.47 to 102.38 % in AuNU compared to Ref, and the sample with sea urchin-shaped gold nanoparticles showed better tensile strength than the spherical gold nanoparticles. The results of measuring the tensile strength of the prepared contact lens samples are shown in Fig. 3.
3.1.4. Contact angle
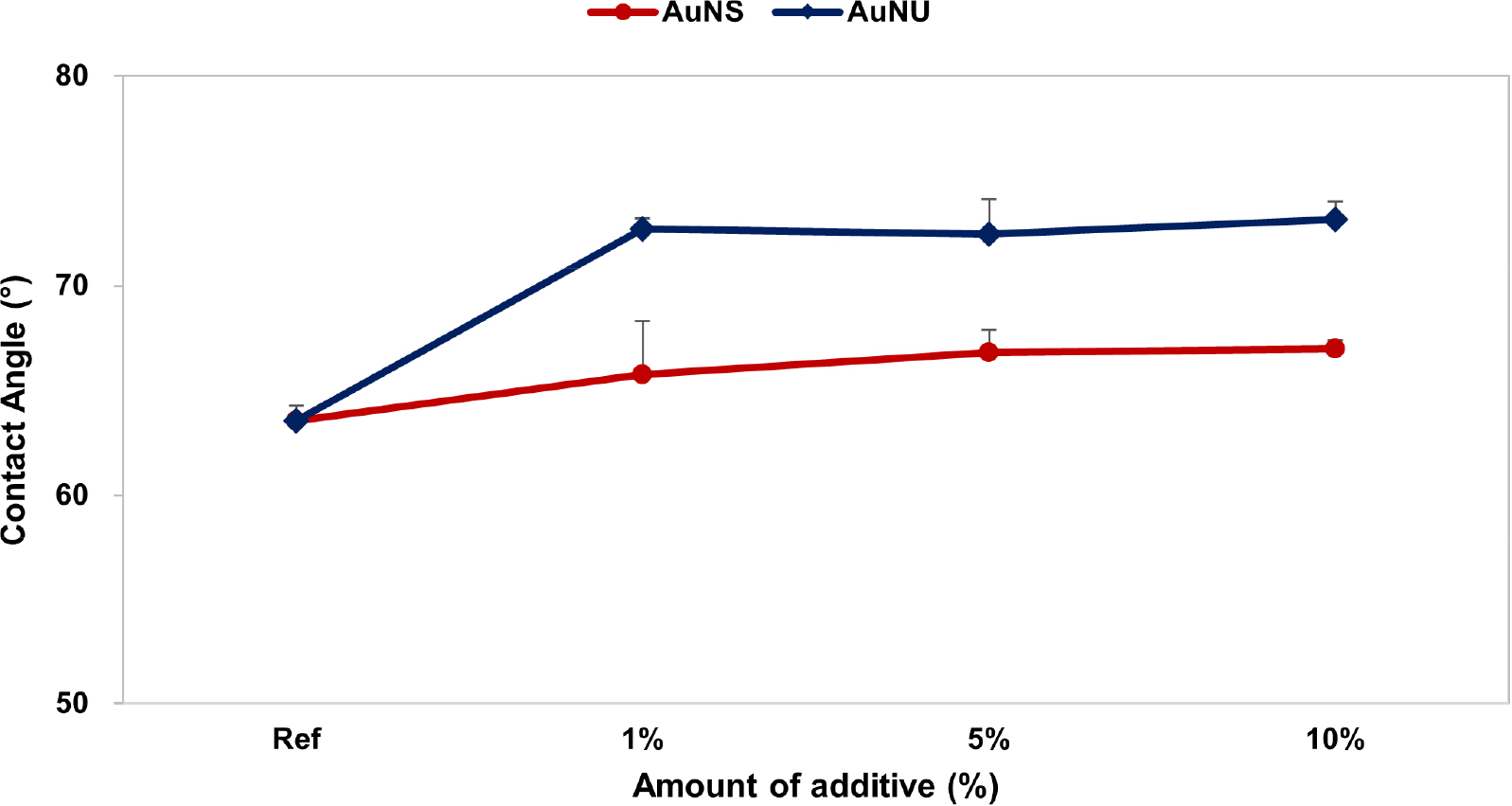
The contact angle was measured to evaluate the wettability of the prepared contact lens, and the average contact angle of Ref was 63.55°. The average contact angle of the samples containing AuNS by ratio was 65.75~67.00°, and the average contact angle of the samples containing AuNU by ratio was 72.45~73.17°. The addition of gold nanoparticles increased the contact angle of the lens, and the sample to which AuNU was added slightly increased the contact angle of the contact lens compared to AuNS, resulting in decreased wettability. The measurement results of the contact angle are shown in Fig. 4.
3.1.5. AFM and SEM analyses
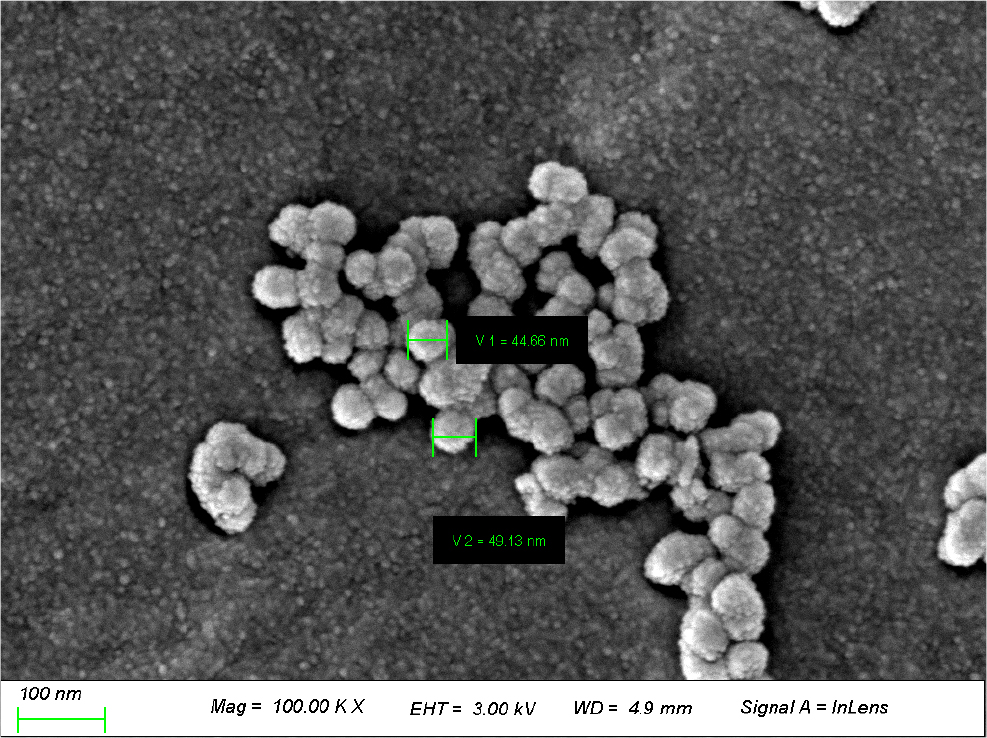
An AFM was used to measure the surface roughness of the contact lens. As a result of measuring the average surface roughness value (Ra) of the prepared lens, Ref was 2.202 nm, AuNS was 2.173 nm, and AuNU was 2.498 nm. In the case of the AuNU group, a sample to which sea urchin-shaped nanoparticles were added, it was found that the surface roughness of the lens slightly increased. It is judged that the shape of the gold nanoparticles had an effect. In addition, SEM was used to confirm the shape of sea urchin-shaped nanoparticles (AuNU). As a result of SEM measurement, it was confirmed that gold nanoparticles with a size of 40 to 50 nm with a sea urchin shape were distributed on the surface of the contact lens. A SEM image of the sample containing sea urchin-shaped nanoparticles is shown in Fig. 5.
3.2. Properties based on surface-modified gold nanoparticles
3.2.1. Optical and physical properties
As a result of measuring the optical and physical properties of the SMNU sample using the surface modification method, it was found that there was no significant difference from the results of the optical transmittance, refractive index, and water content of the sample of AuNU without surface modification. It is judged that the surface modification of gold nanoparticles does not affect basic optical and physical properties such as optical transmittance, refractive index, and water content of the manufactured lens.
3.2.2. Surface properties
As a result of measuring the contact angle of the SMNU sample using the surface modification method, it was found that SMNU1 was 42.85°, SMNU5 was 31.92°, and SMNU10 was 18.64°. SMNU was prepared with hydrophilic modification of the surface of AuNU with low wettability, and it was confirmed that the contact angle of the contact lens prepared using this decreased as the proportion of nanoparticles increased, thereby improving wettability. The measurement results of the contact angle of each sample are shown in Fig. 6.
In addition, as a result of analyzing the surface roughness of the manufactured lens, the surface roughness value (Ra) of the SMNU was 1.767 nm, indicating that the surface roughness was slightly reduced compared to that of the AuNU. It is judged that the surface roughness of the manufactured lens has a great influence on the surface contact angle. Fig. 7 shows an AFM image of each sample according to the surface modification.
3.2.3. Polymerization stability
Polymerization stability is very important because contact lenses are in direct contact with the cornea, and absorbance was measured during the eluate test to confirm the polymerization stability of the prepared sample. As a result of the measurement, AuNU was 0.080 and SMNU was 0.018. In the case of the SMNU sample to which surface modification was applied, the absorbance result was very low compared to the AuNU sample. Consequently, surface modification is judged to improve the stability of the contact lens. The results of the absorbance measurement are shown in Fig. 8.
4. Discussion
As a result of using gold nanoparticles in contact lens materials, it was found that the tensile strength of contact lenses was greatly improved. In particular, sea urchin-shaped gold nanoparticles (AuNU) showed superior tensile strength compared to spherical gold nanoparticles, but reduced wettability and slightly increased the contact angle compared to Ref. In general, the wettability of the lens is proportional to the water content,13) but all samples to which sea urchin-shaped gold nanoparticles were added showed a slight decrease in wettability while maintaining the water content. It is judged that the surface roughness of the contact lens increased due to the shape of the sea urchin-shaped gold nanoparticles. To compensate for this, the surface of sea urchin-shaped gold nanoparticles was modified to be hydrophilic and applied as a contact lens material. As a result, it was found that the addition of surface-modified gold nanoparticles significantly reduced the contact angle of the lens and did not affect the water content. Patients with dry eye syndrome feel rather dry due to osmotic pressure in the case of wearing contact lenses with high water content.14) Patients with dry eye syndrome might experience discomfort when wearing high water content lenses due to osmotic imbalances.14) Since SMNU, a surface-modified sea urchin-shaped nanoparticle, improves wettability without affecting the water content of the lens, it was confirmed that it can be used as a material with a high wettability function while maintaining the water content. In addition, the tensile strength of the lens is excellent, so it is expected to be used extensively in a variety of ways.
CTAB is known to be cytotoxic, but CTAB combined with Au nanoparticles was found to not cause toxicity,15) and in this study, surface-modified contact lenses using CTAB also showed improved polymerization stability compared to conventional contact lenses.
5. Conclusion
After gold nanoparticles of different shapes were prepared for contact lenses, their properties were evaluated. As a result of the measurement, it was found that the gold nanoparticles did not significantly affect the optical transmittance, refractive index, and water content of the contact lens, and increased the tensile strength according to the addition ratio. In particular, sea urchin-shaped gold nanoparticles have very improved tensile strength and have been shown to increase surface roughness and contact angle. Therefore, sea urchin-shaped gold nanoparticles were used for surface modification using a cationic surfactant, and then used for contact lenses. As a result, it was found that the surface-modified gold nanoparticles did not affect the other basic properties of the contact lens and greatly improved wettability at the same time. Therefore, it is judged that nanoparticles produced using various types of gold nanoparticles and surface modification methods can be used as functional materials with high durability and high wettability.