1. Introduction
2. Experimental Procedure
2.1. Measuring particle size and zeta potential
2.2. E. coli and HAp adsorption experiment
3. Results and Discussion
3.1. Measure the particle size
3.2. Measure the PH
3.3. Measure the zeta potential
3.4. E. coli and HAp adsorption experiment
3.5. Antibacterial properties
3.6. Adsorption mechanism of E. coli
4. Conclusion
1. Introduction
Escherichia coli (E. coli) is a facultative anaerobic, Gram-negative, rod-shaped (length: 1~4 µm, width: 0.4~0.7 µm) bacterium commonly found in the small intestine of warm-blooded organisms.1,2,3)Although most strains of E. coli are harmless, some serotypes can cause serious food poisoning in humans. E. coli is absorbed in food, and ingestion of enteropathogenic strains can cause adverse effects in individuals with weakened immune systems and cause infectious diarrhea. E. coli is usually found in the intestines of humans and animals, especially in high concentrations in the large intestine where it is not pathogenic, but when found in other organs, it causes cystitis, pyeloneitis, peritonitis, and sepsis.4,5,6,7,8,9,10,11,12) Hydroxyapatite (HAp) was used to eliminate pathogenic E. coli HAp has a chemical formula of Ca5(PO4)3(OH), similar to the molecular composition of bone material, and has excellent in vivo stability.13,14,15) Because HAp has a positive charge on E. coli, they attract each other through electrostatic interactions. Additionally, because the surface of HAp is porous, it enters the pores of the HAp surface and removes E. coli from the environment.16,17) To elucidate the mechanism by which HAp traps E. coli, it would be useful to visually inspect it under a microscope. To evaluate the antibacterial effect of HAp against E. coli in this study, we investigated the mechanism of E. coli elimination by HAp. Because HAp and E. coli have opposite charges, they attract each other through electrostatic interactions.18,19) Additionally, because the surface of HAp is porous, it enters the pores of the HAp surface and removes E. coli from the environment. These factors are thought to influence the E. coli removal process.20)
2. Experimental Procedure
2.1. Measuring particle size and zeta potential
HAp (0.4 g) was added to 200 mL of water, and the HAp solution was treated using a natural decomposition mill for 24 h. E. coli was cultured in 100 mL of liquid medium at 36.5°C for 24 h. 1 mL of cultured E. coli was added to 50 mL of distilled water. Particle size analysis of the HAp/E. coli mixture was performed under the assumption that the two individual components were the same size. The size change of the 1:1 volume ratio mixture of E. coli and HAp was measured as a function of time (immediately after mixing, 30 and 60 min after mixing). The zeta potential of a mixture of HAp and E. coli of similar size was measured using Otsuka Model ELS-Z at these different time points.
2.2. E. coli and HAp adsorption experiment
Bulk HAp powder was administered to E. coli, and the interaction between E. coli and the HAp surface was visualized using an optical microscope, and continuous images were taken for 30 s using a camera attached to the microscope. To better observe cell flow, the negatively charged surface of E. coli was stained using positively charged crystal violet. E. coli was stained using the Gram stain method and spread on a glass slide. After dropping HAp powder on the slide, the change in distance between E. coli and HAp was observed.
3. Results and Discussion
To explain the removal mechanism of E. coli, HAp particle size, pH, and zeta potential were measured, and the interaction of HAp with E. coli of different particle sizes, pH, and zeta potentials was evaluated, and to explain the interaction mechanism between HAp and E. coli The antibacterial effect of HAp against E. coli was investigated.
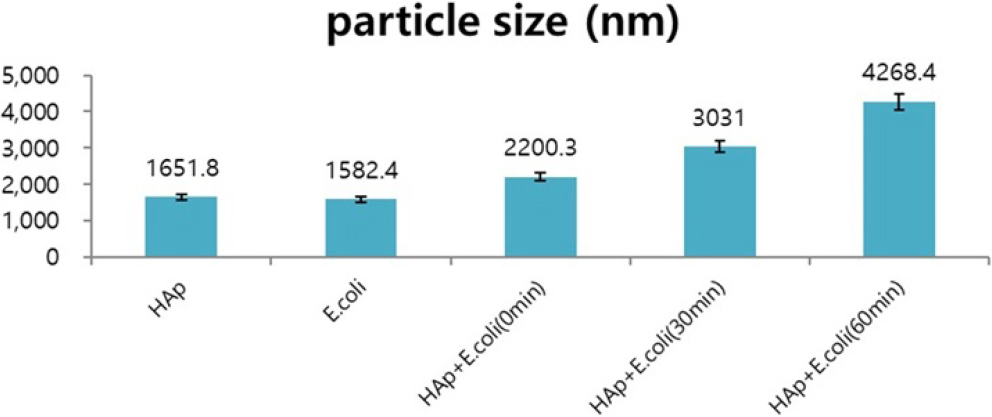
3.1. Measure the particle size
The average size of the prepared HAp powder was confirmed to be 1.65 µm, and the average particle size of E. coli was confirmed to be 1.58 µm. At this time, HAp and E. coli dispersed in water were mixed at a 1:1 volume ratio and the change in particle size over time was measured (Fig. 1). The experiment was repeated five times. After about 1 h, the particle size increased threefold. This result is thought to be because E. coli and HAp come together due to the attractive force generated from the positively charged HAp and the negatively charged E. coli, increasing the particle size.
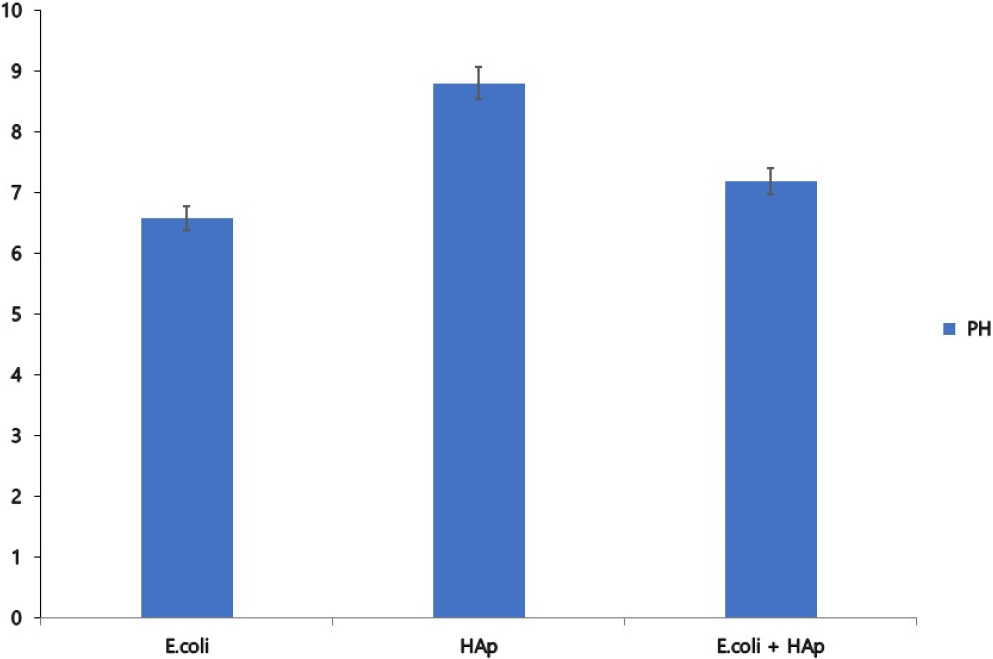
3.2. Measure the PH
The results of measuring the pH of HAp, E. coli, and the mixture are shown in Fig. 2. The pH of E. coli was measured at 6.59 and confirmed as acidic, the pH of HAp was measured at 8.81 and confirmed as basic, and the pH of the mixture was confirmed as neutral at 7.2.
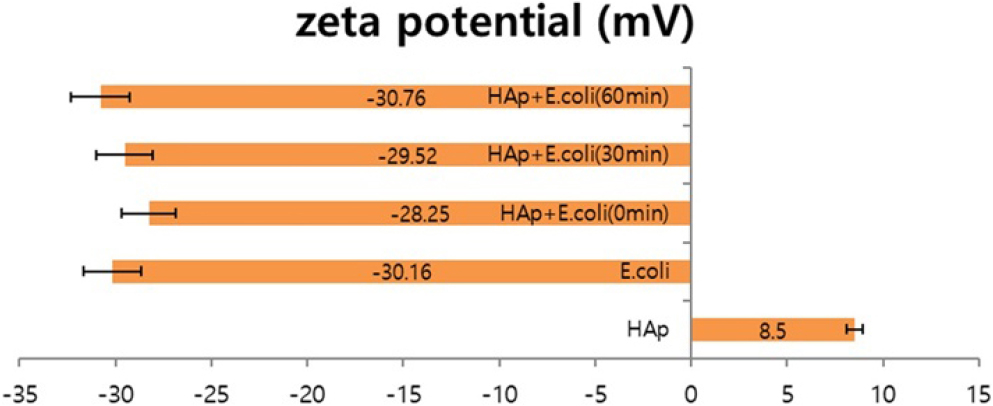
3.3. Measure the zeta potential
The change in zeta potential of E. coli and HAp mixed at a ratio of 1:0.5 was measured at 30 min intervals (Fig. 3). The experiment was repeated five times. At this time, the zeta potential values of E. coli and HAp are -30.16 mV and +8.5 mV, respectively. In addition, as a result of measuring the zeta potential value over time after mixing E. coli and HAp, the zeta potential value of the mixed particles initially decreased compared to the absolute zeta potential value of E. coli and then decreased for about 1 h. When E. coli and HAp are first mixed, a part of E. coli is adsorbed to HAp, reducing zeta potential. After more than 30 min, E. coli is completely adsorbed on the HAp surface, and it is thought that only the zeta potential of E. coli is measured. It was found that Afterwards, -30.76 mV, similar to the absolute zeta potential value of E. coli, can be seen. These results show that in the case of zeta potential, when HAp and E. coli of different polarities are initially mixed, an aggregation reaction occurs due to attractive forces, resulting in a decrease in the zeta potential value. After the agglutination reaction continues, E. coli gather around the HAp. This is thought to have a value similar to the absolute zeta potential value of E. coli.
3.4. E. coli and HAp adsorption experiment
The surface of HAp and the E. coli adsorbed surface HAp were observed using a scanning electron microscope (SEM). As shown in Fig. 4, HAp was confirmed to have a porous surface. And it was observed in the photo that rod-sHAped E. coli was adsorbed on the surface of the porous HAp. In addition, when porous HAp sol was administered to live E. coli in the culture medium, the changing behavior of E. coli stained purple with crystal violet was observed, and the adsorption process of E. coli was continuously observed. The results of continuous high-speed imaging of optical microscope images at 4,000× magnification are shown in Fig. 5. It was observed that the purple E. coli that had spread in the culture medium moved toward the black porous HAp particles over time.
3.5. Antibacterial properties
The antibacterial activity test results are shown in Table 1. The test results are evaluated according to the requirements of the Korean Standards Association (KS K 0693:2011). Among the results for E. coli ATCC 25922, it showed 99.9 % antibacterial activity. Compared to the control sample, which showed an increase in the number of colonies, HAp particles showed a decrease in the number of E. coli colonies.
Table 1.
The result of antibacteria test.
| Test item | Test results | ||
| BLANK | After adding HAp | ||
|
Escherichia coli ATCC 25922 | Initial bacterial content | 2.2 × 104 | 2.2 × 104 |
| 18 h after bacterial reduction | 3.7 × 107 |
<10 99.9 % | |
3.6. Adsorption mechanism of E. coli
Based on the above experimental results, we would like to propose a possible antibacterial mechanism of HAp against E. coli (Fig. 6). When an HAp platform with larger pores than E. coli particles is manufactured and brought into contact with E. coli, the zeta potentials of HAp and E. coli become opposite, so HAp attracts E. coli through electrostatic interaction and adsorbs to the surface of the porous particle, creating an acidic form. It is believed that it is possible to adsorb and remove E. coli using basic HAp.
4. Conclusion
To demonstrate the E. coli removal mechanism, the particle size, pH, and zeta potential of HAp and E. coli were measured, and the particle size, pH, and zeta potential of HAp were measured to evaluate the interaction between E. coli and HAp. A mixture of HAp and E. coli It was confirmed that the particle size of the mixture of HAp and E. coli increased more than three times over time compared to the initial particle size. Afterwards, the pH of the mixture of E. coli and HAp was neutral at 7.2. In addition, the zeta potential value of the mixture of HAp and E. coli was -30.76 mV, similar to the absolute zeta potential value of E. coli after 1 h. Through the experiment, adsorption occurred by attraction between HAp and E. coli with different charges, and the particle size increased as E. coli was adsorbed on the surface of HAp. The adsorption amount increased over time, and the zeta potential value tended to be similar to that of E. coli. This phenomenon is thought to be similar to E. coli as E. coli adheres to the HAp surface over time. E. coli stained with crystal violet was spread on a glass slide and HAp porous sol powder was dropped to confirm the change. As a result, it was observed that more E. coli moved toward the HAp particles over time and was eventually removed. In addition, 99 % of the antibacterial effect was confirmed as a result of examining the antibacterial effect of HAp on E. coli. The adsorption reaction was caused by the zeta potential of HAp and E. coli, and the antibacterial property of removing acidic E. coli from the basic porous surface of HAp was confirmed. These experimental results are expected to suggest a new direction for antibacterial substances using HAp. In addition, it is expected that it will be possible to manufacture antibacterial products that are harmless to the human body. In addition, like the E. coli used in this experiment, Pseudomonas aeruginosa and Listeri also have a surface charge similar to that of E. coli, so it is believed that these bacteria can be adsorbed and removed using HAp.